A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization - PubMed (original) (raw)
A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization
Renqiang Liu et al. Antiviral Res. 2018 Feb.
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) has been a highly threatening zoonotic pathogen since its outbreak in 2012. Similar to SARS-CoV, MERS-CoV belongs to the coronavirus family and can induce severe respiratory symptoms in humans, with an average case fatality rate of 35% according to the World Health Organization. Spike (S) protein of MERS-CoV is immunogenic and can induce neutralizing antibodies, thus is a potential major target for vaccine development. Here we constructed a chimeric virus based on the vesicular stomatitis virus (VSV) in which the G gene was replaced by MERS-CoV S gene (VSVΔG-MERS). The S protein efficiently incorporated into the viral envelope and mediated cell entry through binding its receptor, human DPP4. Knockdown of clathrin expression by siRNA drastically abrogated the infection of VSVΔG-MERS in Vero cells. Furthermore, in animal studies, the recombinant virus induced neutralizing antibodies and T cell responses in rhesus monkeys after a single intramuscular or intranasal immunization dose. Our findings indicate the potential of the chimeric VSVΔG-MERS as a rapid response vaccine candidate against emerging MERS-CoV disease.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
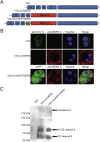
Fig. 1
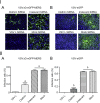
Generation of VSVΔG-MERS and VSVΔG-eGFP-MERS and expression of S protein. Schematic representation of the recombinant viruses (A). S protein expression in VSVΔG-MERS and VSVΔG-eGFP-MERS infected Vero E6 cells by indirect immunofluorescence staining (B) and Western blot (C).
Fig. 2
Growth properties of recombinant viruses in Vero E6 cells. VSV, VSVΔG-MERS and VSVΔG-eGFP-MERS were inoculated on Vero E6 cells on 6-well plate at a MOI = 0.01, supernatant was taken out from 12 h to 96 h at 12-h intervals. The titer was expressed as the reciprocal of the highest dilution titer (fluorescence forming unit, FFU).
Fig. 3
Incorporation of S protein into viral particles. S protein efficiently incorporated into the viral particles as indicated by electron and immunoelectron microscopy (A). VSV-MERS, a recombinant VSV virus has MERS-CoV S gene inserted between VSV M and G gene as an additional transcription unit (unpublished work). VSV-MERS has both G and S protein on viral surface. Long arrows indicate S protein; short stealth arrows indicate VSV G protein.
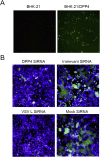
Fig. 4
VSVΔG-MERS and VSVΔG-eGFP-MERS utilize human DPP4 as receptor. BHK-21 cells and human DPP4-transfected BHK-21 cells were infected with VSVΔG-eGFP-MERS (A). Vero E6 cells transfected with DPP4 siRNA, irrelevant siRNA, VSV L siRNA and mock siRNA were infected with VSVΔG-eGFP-MERS (B).
Fig. 5
Clathrin is important for VSVΔG-eGFP-MERS infection. Vero E6 cells were transfected with clathrin siRNA, VSV L siRNA, mock siRNA and irrelevant siRNA to observe the impact of clathrin-knockdown on VSVΔG-eGFP-MERS (I A) or VSV-eGFP (I B) infection. The infection ratio (mean ± SD) and statistical analysis is presented (II A and B); significant differences between conditions is designated with (a) in panel II A and (b) in panel II B; p < .01.
Fig. 6
Humoral responses of VSVΔG-MERS-immunized mice. 10 mice were intramuscularly immunized with 1 × 106 FFU VSVΔG-MERS, mice were observed and weighed daily for 14 days (A). At 21 days after the first dose, mice were given the booster dose. S protein specific IgG (B) and neutralizing antibody (C) was analyzed. (a), (b) p < .01.
Fig. 7
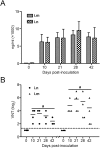
Humoral immune responses of rhesus monkeys to VSVΔG-MERS vaccination. Monkeys were immunized with 2 × 107 FFU of recombinant virus intramuscularly (i.m) or intranasally (i.n). Blood samples were collected at the indicated time-points. Serum IgG (A) and neutralizing antibody (B) were determined. The neutralizing antibody from both routes at the same time-point was compared. (a) p < .01.
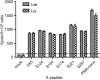
Fig. 8
T cell responses of immunized rhesus monkeys to VSVΔG-MERS vaccination. PBMCs from immunized monkeys were tested for MERS-CoV S peptide-specific T cell responses by ELISPOT. An S protein overlapping peptide pool which contained 269 peptides (15-mers designed for CD8+ T cells) were used to stimulate the monkey PBMCs. Ex-vivo IFN-γ ELISPOT assay was performed to determine the active cells. Six peptides that yielded the most spots were selected for presentation. No statistical difference was observed in the peptides between the i.m and i.n groups.
Similar articles
- Single intranasal immunization with chimpanzee adenovirus-based vaccine induces sustained and protective immunity against MERS-CoV infection.
Jia W, Channappanavar R, Zhang C, Li M, Zhou H, Zhang S, Zhou P, Xu J, Shan S, Shi X, Wang X, Zhao J, Zhou D, Perlman S, Zhang L. Jia W, et al. Emerg Microbes Infect. 2019;8(1):760-772. doi: 10.1080/22221751.2019.1620083. Emerg Microbes Infect. 2019. PMID: 31130102 Free PMC article. - Characterization of the Immune Response of MERS-CoV Vaccine Candidates Derived from Two Different Vectors in Mice.
Li E, Yan F, Huang P, Chi H, Xu S, Li G, Liu C, Feng N, Wang H, Zhao Y, Yang S, Xia X. Li E, et al. Viruses. 2020 Jan 20;12(1):125. doi: 10.3390/v12010125. Viruses. 2020. PMID: 31968702 Free PMC article. - Inactivated Rabies Virus Vectored MERS-Coronavirus Vaccine Induces Protective Immunity in Mice, Camels, and Alpacas.
Chi H, Wang Y, Li E, Wang X, Wang H, Jin H, Han Q, Wang Z, Wang X, Zhu A, Sun J, Zhuang Z, Zhang L, Ye J, Wang H, Feng N, Hu M, Gao Y, Zhao J, Zhao Y, Yang S, Xia X. Chi H, et al. Front Immunol. 2022 Jan 31;13:823949. doi: 10.3389/fimmu.2022.823949. eCollection 2022. Front Immunol. 2022. PMID: 35173733 Free PMC article. - Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development.
Mubarak A, Alturaiki W, Hemida MG. Mubarak A, et al. J Immunol Res. 2019 Apr 7;2019:6491738. doi: 10.1155/2019/6491738. eCollection 2019. J Immunol Res. 2019. PMID: 31089478 Free PMC article. Review. - Progress of Middle East respiratory syndrome coronavirus vaccines: a patent review.
Choi J, Kim MG, Oh YK, Kim YB. Choi J, et al. Expert Opin Ther Pat. 2017 Jun;27(6):721-731. doi: 10.1080/13543776.2017.1281248. Epub 2017 Jan 25. Expert Opin Ther Pat. 2017. PMID: 28121202 Review.
Cited by
- Vaccination strategies to combat novel corona virus SARS-CoV-2.
Pandey SC, Pande V, Sati D, Upreti S, Samant M. Pandey SC, et al. Life Sci. 2020 Sep 1;256:117956. doi: 10.1016/j.lfs.2020.117956. Epub 2020 Jun 12. Life Sci. 2020. PMID: 32535078 Free PMC article. Review. - Small GTPase-A Key Role in Host Cell for Coronavirus Infection and a Potential Target for Coronavirus Vaccine Adjuvant Discovery.
Hou W, Wang S, Wu H, Xue L, Wang B, Wang S, Wang H. Hou W, et al. Viruses. 2022 Sep 14;14(9):2044. doi: 10.3390/v14092044. Viruses. 2022. PMID: 36146850 Free PMC article. Review. - An overview of Middle East respiratory syndrome coronavirus vaccines in preclinical studies.
Zhang N, Shang J, Li C, Zhou K, Du L. Zhang N, et al. Expert Rev Vaccines. 2020 Sep;19(9):817-829. doi: 10.1080/14760584.2020.1813574. Epub 2020 Sep 8. Expert Rev Vaccines. 2020. PMID: 32842811 Free PMC article. Review. - Vaccine development for zoonotic viral diseases caused by positive‑sense single‑stranded RNA viruses belonging to the Coronaviridae and Togaviridae families (Review).
Babaeimarzangou SS, Zaker H, Soleimannezhadbari E, Gamchi NS, Kazeminia M, Tarighi S, Seyedian H, Tsatsakis A, Spandidos DA, Margina D. Babaeimarzangou SS, et al. Exp Ther Med. 2022 Nov 30;25(1):42. doi: 10.3892/etm.2022.11741. eCollection 2023 Jan. Exp Ther Med. 2022. PMID: 36569444 Free PMC article. Review. - Air-liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges and future directions.
Baldassi D, Gabold B, Merkel O. Baldassi D, et al. Adv Nanobiomed Res. 2021 May 6;1(6):2000111. doi: 10.1002/anbr.202000111. eCollection 2021 Jun. Adv Nanobiomed Res. 2021. PMID: 34345878 Free PMC article.
References
- Annan A., Baldwin H.J., Corman V.M., Klose S.M., Owusu M., Nkrumah E.E., Badu E.K., Anti P., Agbenyega O., Meyer B., Oppong S., Sarkodie Y.A., Kalko E.K., Lina P.H., Godlevska E.V., Reusken C., Seebens A., Gloza-Rausch F., Vallo P., Tschapka M., Drosten C., Drexler J.F. Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg. Infect. Dis. 2013;19:456–459. - PMC - PubMed
- Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370:2499–2505. - PubMed
- de Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L., Fischer E.R., Martellaro C., Okumura A., Chang J., Scott D., Benecke A.G., Katze M.G., Feldmann H., Munster V.J. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16598–16603. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous