YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration - PubMed (original) (raw)
. 2018 Jan 4;22(1):35-49.e7.
doi: 10.1016/j.stem.2017.11.001. Epub 2017 Dec 14.
Luca Azzolin 2, Martti Maimets 1, Marianne Terndrup Pedersen 1, Robert P Fordham 3, Stine L Hansen 1, Hjalte L Larsen 1, Jordi Guiu 1, Mariana R P Alves 1, Carsten F Rundsten 1, Jens V Johansen 1, Yuan Li 4, Chris D Madsen 5, Tetsuya Nakamura 6, Mamoru Watanabe 6, Ole H Nielsen 4, Pawel J Schweiger 1, Stefano Piccolo 7, Kim B Jensen 8
Affiliations
- PMID: 29249464
- PMCID: PMC5766831
- DOI: 10.1016/j.stem.2017.11.001
YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration
Shiro Yui et al. Cell Stem Cell. 2018.
Abstract
Tissue regeneration requires dynamic cellular adaptation to the wound environment. It is currently unclear how this is orchestrated at the cellular level and how cell fate is affected by severe tissue damage. Here we dissect cell fate transitions during colonic regeneration in a mouse dextran sulfate sodium (DSS) colitis model, and we demonstrate that the epithelium is transiently reprogrammed into a primitive state. This is characterized by de novo expression of fetal markers as well as suppression of markers for adult stem and differentiated cells. The fate change is orchestrated by remodeling the extracellular matrix (ECM), increased FAK/Src signaling, and ultimately YAP/TAZ activation. In a defined cell culture system recapitulating the extracellular matrix remodeling observed in vivo, we show that a collagen 3D matrix supplemented with Wnt ligands is sufficient to sustain endogenous YAP/TAZ and induce conversion of cell fate. This provides a simple model for tissue regeneration, implicating cellular reprogramming as an essential element.
Keywords: YAP/TAZ; intestinal stem cells; mechano-sensing; regeneration; reprogramming.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
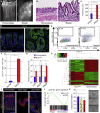
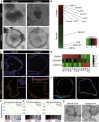
Repairing Intestinal Epithelium Has a Cellular and Molecular Profile Distinct from the Normal Epithelium (A) Macroscopic images of the distal part of the colon in an untreated mouse (left) and a mouse 2 weeks post-administration of DSS (right). The demarcated area indicates regions undergoing active re-epithelialization. Scale bar, 2 mm. (B) H&E staining of homeostatic tissue and tissue in the repair phase at 2 weeks following DSS administration. Scale bar, 100 μm. (C) The mucosal/submucosal thickness at homeostasis and repair phase (2 weeks). Shown are mean distances ± SEM (n = 4 animals; p = 0.007 based on two-sided Student’s t test). (D) Sca1 (green) expression in colonic epithelium in homeostatic and repair phase. Sections are counterstained with DAPI (blue). The demarcated line indicates the epithelial structure. Scale bar, 100 μm. (E) Flow cytometric analysis of cells from the colonic epithelium during homeostasis and in repair phase (2 weeks post-administration of DSS). Diagrams show representative plots for Sca1-PECy5 in live CD45−veCD31−veEpcam+ve cell population. (F) Quantification of the percentages of Sca1high cells in homeostasis and repair phase. Diagram shows average ± SEM (n = 3; p = 4 × 10−5 based on two-sided Student’s t test). (G) qRT-PCR analyses in Epcam+Sca1high cells sorted via FACS from the repairing epithelium and in homeostatic epithelial cells (Epcam+). Bars represent average levels ± SEM (n = 3; Ly6a, p = 1.3 × 10−4; Reg3b, p = 9.7 × 10−3; Reg3g, p = 8.5 × 10−3 based on two-sided Student’s t test). (H) Heatmap analysis of differentially expressed probe sets (fold change > 2.0; FDR < 0.05), comparing the expression profile of epithelial cells isolated from homeostatic tissue and Sca1high cells from the repairing epithelium. (I) Detection of Lrig1 (green) in homeostasis, early and late repair phase counterstained for E-cadherin (red) with DAPI (blue). Scale bar, 50 μm. (J) GSEA of repairing and homeostatic epithelium using Lgr5 intestinal stem cell gene signature. (K) Organoid formation for Epcam+ cells isolated from normal homeostasis, as well as Epcam+ Sca1low and Sca1high cells during tissue repair showing representative images of formed organoids. Bars represent average number of organoids formed ± SEM (n = 3 animals). See also Figure S1 and Table S1.
Figure 2
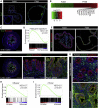
The Repairing Epithelium Adopts a Fetal-like Expression Profile (A) Sca1 (green) expression in organoids from adult animals (Adult) and in fetal organoids (Fetal) derived from the proximal small intestine. Insets show the enlarged view of the indicated region. Images are counterstained with DAPI (blue). Scale bar, 50 μm. (B) Heatmap of differentially expressed probe sets showing genes upregulated more than 2-fold comparing adult organoids (green) and fetal organoids (red) (n = 3; fold change > 2; FDR < 0.05). Multiple members of Ly6 and Annexin gene families are upregulated in the fetal cells. (C) Sca1 (green) expression in fetal colon at embryonic day (E)16.5 counterstained with EpCAM (red) and DAPI (blue). Scale bars, 50 μm. (D) GSEA showing enrichment of the fetal intestinal gene signature in the repairing epithelium. (E) Anxa1 (green) expression in adult organoids (Adult) and fetal organoids (Fetal) derived from the proximal small intestine. Images are counterstained with E-cadherin (red) and DAPI (blue). Scale bar, 100 μm. (F) Anxa1 (green) expression in fetal colon at E16.5, colon in homeostasis and in the repair phase. Images are counterstained with E-cadherin (red) and DAPI (blue). Scale bars, 50 μm for fetal colon and 100 μm for adult colon. (G) ANXA1 (green) in colonic biopsy from non-inflamed and inflamed regions obtained from a patient with ulcerative colitis. Images are counterstained with E-cadherin (red) and DAPI (blue). Scale bar, 100 μm. (H and I) GSEAs showing enrichment of the transcriptional signatures for the mouse repairing epithelium (H, mRepair) and mouse fetal state (I, mFetal) in samples isolated from patients with active colitis (hColitis) compared to control individuals (hNormal). See also Figure S2 and Table S2.
Figure 3
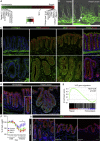
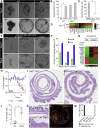
The Repairing Epithelium Displays Features of Active Mechano-Transduction (A) Differentially expressed probes ranked in heatmap comparing tissue homeostasis and repair phase (n = 3; 2-fold regulated; FDR < 0.05). Selected genes associated with lineage differentiation and extracellular matrix interactions are indicated. (B) Second harmonic generation (Collagen; green) overlapped with bright-field microscopy in tissue sections from homeostasis and repair phase. Demarcated lines indicate crypts and muscularis mucosa. Arrows indicate collagen fibers encapsulating _de novo_-formed crypts. Scale bar, 50 μm. (C) Detection of β1 integrin, FAK, pSrc, and Phalloidin (green) during the repair phase and homeostasis. Images are counterstained with E-cadherin (red) and DAPI (blue). Scale bars, 100 μm for β1 integrin, FAK, and pSrc and 50 μm for Phalloidin. (D) Detection of YAP (green) during the repair phase and homeostasis. Inset shows enlarged view of a single colonic crypt during homeostasis. Sections were counterstained with E-cadherin (red) and DAPI (blue). Scale bars, 100 μm. (E) GSEA showing enrichment of a YAP gene signature in repairing relative to normal colonic epithelium. (F) Weight curves for animals treated with DSS and subsequently with either vehicle or FAK and Src inhibitors during tissue repair. Individual points represent the average weight relative to the starting point ± SEM (n = 5 for all groups). Significance was assessed using a two-way ANOVA with Bonferroni correction for multiple comparisons (Srcinhib versus Ctrl day 9, p = 0.02; day 10, p = 0.004; day 11, p = 0.03; and day 12, p = 0.01; FAKinhib versus Ctrl day 9, p = 4 × 10−4; day 10, p = 2 × 10−4; day 11, p = 10−4; and day 12, p < 10−4). (G) Detection of YAP (green) during the repair phase (day 12) in vehicle and Srcinhibitor- and FAKinhibitor-treated animals. Sections were counterstained with E-cadherin (red) and DAPI (blue). Scale bars, 50 μm. See also Figure S3.
Figure 4
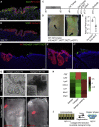
Rebuilding the Repairing Epithelium In Vitro Based on Defined Components (A) Murine small intestinal epithelial cells cultured in the presence of EGF, Noggin, and R-spondin1 (ENR) or with the addition of Wnt3a (+Wnt3a) in either Matrigel or collagen type I. Scale bars, 100 μm. (B) Heatmap of differentially expressed probe sets between culture conditions using MG/ENR+Wnt3a (ENRW, green) and COL/ENRW (red) (n = 6; fold change > 1.5; FDR < 0.1). Examples of differentially expressed genes are indicated. (C) Detection of F-actin with Phalloidin (gray) counterstained with DAPI (blue) and YAP (green) counterstained with E-cadherin (red) and DAPI (blue) in Matrigel and collagen type I cultures from the small intestine. Insets show enlarged view of the indicated regions. Scale bars, 50 μm in the top panels and 100 μm in the bottom panels. (D) Heatmap of Z score-transformed relative expression levels as determined by qPCR for selected YAP/TAZ target genes and markers of the fetal and adult states in cultures of small intestinal epithelial cells grown in either Matrigel (MG) or collagen type I (Col) in the presence of EGF/Noggin/Rspondin (ENR) with or without Wnt3a (W). (E) Detection of Sca1 (green, left) and Anxa1 (green, right) in collagen type I cultures from the small intestine. Inset shows enlarged view of the region indicated. Images are counterstained with DAPI (blue) and image for Anxa1 is additionally counterstained with E-cadherin (red). Scale bars, 50 μm. (F) GSEA showing enrichment of the collagen culture gene signature in the repairing epithelium. (G) GSEA showing enrichment of YAP and fetal gene signatures in ENRW-Collagen relative to ENRW-Matrigel cultures. (H) Mouse and human colonic organoids propagated in collagen type I. For human colonic crypts, Prostaglandin E2 (PGE2) is supplemented. Scale bar, 100 μm. See also Figure S4.
Figure 5
YAP/TAZ Transcriptional Activation Is Required for Cellular Reprogramming (A) Normal organoids (WT) and _Apc_-knockout (_Apc_KO) spheres derived from the small intestine cultured in collagen type I with the indicated cytokine cocktail. Time point of analysis is indicated in the left bar. Scale bar, 100 μm. (B) Quantification of seeding efficiency for WT and _Apc_KO organoids in Noggin and EGF (EN) with or without R-spondin and Wnt3a (RW). Bars represent mean ± SEM (n = 3; EN versus +RW for WT, p = 4.8 × 10−6 based on two-sided Student’s t test). (C) Quantification of seeding efficiency for _Apc_KO organoids in collagen with mevastatin (Mev) and verteporfin (VP). Bars represent mean ± SEM (n = 3; p = 1.8 × 10−5 based on two-sided Student’s t test). (D) Heatmap of Z score-transformed relative expression levels as determined by qPCR for selected YAP/TAZ target genes and markers of the fetal and adult states in _Apc_KO organoid cultures in EGF/Noggin in either Matrigel (MG) or collagen type I (Col). (E) Spheroids derived from the small intestine of Rosa26-rtTA;tetO-YAP-S127A (rtTA/tetOYAP), cultured in collagen type I under the indicated cytokine cocktail ± Doxycycline. Time point of analysis is indicated in the left bar. Scale bar, 100 μm. (F) Quantification of seeding efficiency for organoids from rtTA/tetOYAP cultured with Wnt3a and doxycycline in collagen type I. Additionally, the efficiency is measured in the presence of either Src or FAK inhibitors. Bars represent mean ± SEM (n = 3). (G) Heatmap of Z score-transformed relative expression levels as determined by qPCR for selected YAP/TAZ target genes and markers of the fetal and adult states in rtTA/tetOYAP organoids cultured in matrigel (MG) or collagen type I supplemented with either Wnt3a (no Dox) or Doxycycline (Dox) to induce YAP S127A expression. (H) Weight curve for Yap fl/fl;Taz fl/fl (Ctrl) and Vil-CreERT2;_Yap_fl/fl;_Taz_fl/fl conditional double knockout (cDKO) animals following DSS administration. Mean ± SEM is presented (Ctrl n = 6; cDKO n = 4). The observed differences are significant from day 8 and onward based on two-sided ANOVA with Bonferroni correction for multiple comparisons (day 8, p = 6 × 10−4; day 9 onward, p < 10−4). (I) Representative H&E staining of Swiss roll of DSS-treated colon from Ctrl and cDKO animals at 13 days following the administration of DSS. Scale bar, 1 mm. (J) Quantification of the length of the denuded area in Ctrl and cDKO colons. Data are presented as mean ± SEM (Ctrl n = 6; cDKO n = 4; p = 0.019 with a Mann-Whitney exact two-sided test). (K) Boxed areas from (I) shown in higher magnification. Scale bar, 100 μm. (L) Detection of YAP (green) and Sca1 (red) during the repair phase in tissue from cDKO animals at day 13 following DSS administration. Demarcated lines indicate epithelial cysts without YAP and Sca1 expression. Scale bar, 100 μm. (M) Quantification of YAP/Sca1 status in remodeling crypts and epithelial cysts observed in the cDKO tissue (n = 3 animals; 50–200 cells scored per condition; p < 10−4 based on two-way ANOVA). See also Figure S5.
Figure 6
Injury-Induced Cellular Reprogramming Is Reversible (A and B) Serial sections of engrafted patches from Matrigel cultures analyzed for (A) tdTomato (red) and Sca1 (green) and (B) YAP (green) and E-cadherin (red) 1 day after transplantation (day 12). Scale bar, 100 μm. (C) Diagram of the transplantation strategy using cells from the conditional YAP/TAZ cdKO cells and control animals. Indicated are the administration of DSS (day 0–5), time points for transplantation (days 8 and 11), administration of 4-hydroxy tamoxifen (days 12 and 13), as well as the final analysis (day 16). (D) Whole-mount analysis of the colon for control (tdTomato+/red) and Villin CreER YAP/TAZ cDKO cells (eGFP+/green) before (day 12) and after (day 16) tamoxifen administration. Arrows illustrate areas of GFP+ cells. (E) Quantification of the ratio of the area covered by GFP- versus RFP-expressing cells before and after tamoxifen treatment. Each dot represents independent animals, and data are presented as the mean ± SEM (p = 0.029 based on a Mann-Whitney exact one-sided test). (F) Section of area with engrafted RFP- (red) and GFP- (green) expressing cells. The engraftments are shown at higher magnification in F’ and F”. Scale bar, 100 μm. (G) Small intestinal epithelial cells cultured for 3 passages (P3) in collagen type I with ENRW grow as organoids, when replated in Matrigel (replating passage 3) in the presence of ENR. Inset shows secretory cells in an organoid. Scale bar, 100 μm. (H) Heatmap of Z score-transformed relative expression levels as determined by qPCR for selected various markers expressed by fetal and adult epithelia grown in ENR in MG (Matrigel), then transferred into collagen and cultured in the presence of ENRW (Collagen), and lastly replated in Matrigel and cultured in ENR (Matrigel replating). (I) Engrafted patches (red) from tdTomato-expressing cells cultured for 3 passages in either collagen type I or Matrigel. Scale bars, 2 mm. (J) Model depicting how environmental changes control cellular identity during tissue repair via YAP/TAZ signaling. See also Figure S6.
Comment in
- Colonic epithelium rejuvenation through YAP/TAZ.
Chen R, Guan KL. Chen R, et al. EMBO J. 2018 Jan 17;37(2):164-166. doi: 10.15252/embj.201798618. Epub 2017 Dec 27. EMBO J. 2018. PMID: 29282206 Free PMC article. - Think About the Environment: Cellular Reprogramming by the Extracellular Matrix.
Huels DJ, Medema JP. Huels DJ, et al. Cell Stem Cell. 2018 Jan 4;22(1):7-9. doi: 10.1016/j.stem.2017.12.006. Cell Stem Cell. 2018. PMID: 29304343
Similar articles
- Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes.
Komatsu N, Kajiya M, Motoike S, Takewaki M, Horikoshi S, Iwata T, Ouhara K, Takeda K, Matsuda S, Fujita T, Kurihara H. Komatsu N, et al. Stem Cell Res Ther. 2018 Dec 7;9(1):342. doi: 10.1186/s13287-018-1085-9. Stem Cell Res Ther. 2018. PMID: 30526677 Free PMC article. - YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate.
Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S, Cordenonsi M, Piccolo S. Totaro A, et al. Nat Commun. 2017 May 17;8:15206. doi: 10.1038/ncomms15206. Nat Commun. 2017. PMID: 28513598 Free PMC article. - Think About the Environment: Cellular Reprogramming by the Extracellular Matrix.
Huels DJ, Medema JP. Huels DJ, et al. Cell Stem Cell. 2018 Jan 4;22(1):7-9. doi: 10.1016/j.stem.2017.12.006. Cell Stem Cell. 2018. PMID: 29304343 - YAP/TAZ upstream signals and downstream responses.
Totaro A, Panciera T, Piccolo S. Totaro A, et al. Nat Cell Biol. 2018 Aug;20(8):888-899. doi: 10.1038/s41556-018-0142-z. Epub 2018 Jul 26. Nat Cell Biol. 2018. PMID: 30050119 Free PMC article. Review. - Role of the extracellular matrix and YAP/TAZ in cell reprogramming.
Liu L, Liu M, Xie D, Liu X, Yan H. Liu L, et al. Differentiation. 2021 Nov-Dec;122:1-6. doi: 10.1016/j.diff.2021.11.001. Epub 2021 Nov 3. Differentiation. 2021. PMID: 34768156 Review.
Cited by
- R-spondin/YAP axis promotes gastric oxyntic gland regeneration and Helicobacter pylori-associated metaplasia in mice.
Fischer AS, Müllerke S, Arnold A, Heuberger J, Berger H, Lin M, Mollenkopf HJ, Wizenty J, Horst D, Tacke F, Sigal M. Fischer AS, et al. J Clin Invest. 2022 Nov 1;132(21):e151363. doi: 10.1172/JCI151363. J Clin Invest. 2022. PMID: 36099044 Free PMC article. - A DLG1-ARHGAP31-CDC42 axis is essential for the intestinal stem cell response to fluctuating niche Wnt signaling.
Castillo-Azofeifa D, Wald T, Reyes EA, Gallagher A, Schanin J, Vlachos S, Lamarche-Vane N, Bomidi C, Blutt S, Estes MK, Nystul T, Klein OD. Castillo-Azofeifa D, et al. Cell Stem Cell. 2023 Feb 2;30(2):188-206.e6. doi: 10.1016/j.stem.2022.12.008. Epub 2023 Jan 13. Cell Stem Cell. 2023. PMID: 36640764 Free PMC article. - Claudin-2 protects against colitis-associated cancer by promoting colitis-associated mucosal healing.
Ahmad R, Kumar B, Thapa I, Tamang RL, Yadav SK, Washington MK, Talmon GA, Yu AS, Bastola DK, Dhawan P, Singh AB. Ahmad R, et al. J Clin Invest. 2023 Dec 1;133(23):e170771. doi: 10.1172/JCI170771. J Clin Invest. 2023. PMID: 37815870 Free PMC article. - Molecular Network Analyses Implicate Death-Associated Protein Kinase 3 (DAPK3) as a Key Factor in Colitis-Associated Dysplasia Progression.
Chen HM, MacDonald JA. Chen HM, et al. Inflamm Bowel Dis. 2022 Oct 3;28(10):1485-1496. doi: 10.1093/ibd/izac098. Inflamm Bowel Dis. 2022. PMID: 35604388 Free PMC article. - Activation of the RhoA-YAP-β-catenin signaling axis promotes the expansion of inner ear progenitor cells in 3D culture.
Xia M, Chen Y, He Y, Li H, Li W. Xia M, et al. Stem Cells. 2020 Jul;38(7):860-874. doi: 10.1002/stem.3175. Epub 2020 Mar 26. Stem Cells. 2020. PMID: 32159914 Free PMC article.
References
- Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. - PubMed
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. - PubMed
- Barker N., van Oudenaarden A., Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. - PubMed
- Beaugerie L., Itzkowitz S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015;372:1441–1452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous