The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics - PubMed (original) (raw)
. 2017 Dec 13;46(6):462-472.
doi: 10.1159/000484633. Online ahead of print.
Kenneth W Mahaffey 3, Bruce Neal 1 4 5 6, Rajiv Agarwal 7, George L Bakris 8, Barry M Brenner 9, Scott Bull 10, Christopher P Cannon 11, David M Charytan 12, Dick de Zeeuw 13, Robert Edwards 10, Tom Greene 14, Hiddo J L Heerspink 13, Adeera Levin 15, Carol Pollock 16, David C Wheeler 17, John Xie 10, Hong Zhang 18, Bernard Zinman 19, Mehul Desai 10, Vlado Perkovic 1; CREDENCE study investigators
Collaborators, Affiliations
- PMID: 29253846
- PMCID: PMC5804835
- DOI: 10.1159/000484633
The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics
Meg J Jardine et al. Am J Nephrol. 2017.
Abstract
Background: People with diabetes and kidney disease have a high risk of cardiovascular events and progression of kidney disease. Sodium glucose co-transporter 2 inhibitors lower plasma glucose by reducing the uptake of filtered glucose in the kidney tubule, leading to increased urinary glucose excretion. They have been repeatedly shown to induce modest natriuresis and reduce HbA1c, blood pressure, weight, and albuminuria in patients with type 2 diabetes. However, the effects of these agents on kidney and cardiovascular events have not been extensively studied in patients with type 2 diabetes and established kidney disease.
Methods: The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial aims to compare the efficacy and safety of canagliflozin -versus placebo at preventing clinically important kidney and cardiovascular outcomes in patients with diabetes and established kidney disease. CREDENCE is a randomized, double-blind, event-driven, placebo-controlled trial set in in 34 countries with a projected duration of ∼5.5 years and enrolling 4,401 adults with type 2 diabetes, estimated glomerular filtration rate ≥30 to <90 mL/min/1.73 m2, and albuminuria (urinary albumin:creatinine ratio >300 to ≤5,000 mg/g). The study has 90% power to detect a 20% reduction in the risk of the primary outcome (α = 0.05), the composite of end-stage kidney disease, doubling of serum creatinine, and renal or cardiovascular death.
Conclusion: CREDENCE will provide definitive evidence about the effects of canagliflozin on renal (and cardiovascular) outcomes in patients with type 2 diabetes and established kidney disease.
Trial registration: EudraCT number: 2013-004494-28; ClinicalTrials.gov identifier: NCT02065791.
Keywords: Canagliflozin; Chronic kidney disease; Diabetic nephropathy; Renal outcomes.
© 2017 S. Karger AG, Basel.
Figures
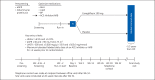
Fig. 1.
Study design. eGFR, estimated glomerular filtration rate; AHA, anti-hyperglycemic agent; BP, blood pressure; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HbA1c, glycated hemoglobin; R, randomization; FPI, first patient in; Wk, week; UACR, urinary albumin:creatinine ratio.
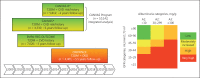
Fig. 2.
Overview of trial timelines. CANVAS, CANagliflozin cardioVascular Assessment Study; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; CANVAS-R, CANagliflozin cardioVascular Assessment Study–Renal; EMPA-REG, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; DKD, diabetic kidney disease; GFR, glomerular filtration rate; CKD, chronic kidney disease. * Note that the patient populations in CANVAS and CANVAS-R are nearly identical to facilitate an integrated analysis of the data.
Similar articles
- Glycemic Control and Effects of Canagliflozin in Reducing Albuminuria and eGFR: A Post Hoc Analysis of the CREDENCE Trial.
van der Hoek S, Jongs N, Oshima M, Neuen BL, Stevens J, Perkovic V, Levin A, Mahaffey KW, Pollock C, Greene T, Wheeler DC, Jardine MJ, Heerspink HJL. van der Hoek S, et al. Clin J Am Soc Nephrol. 2023 Jun 1;18(6):748-758. doi: 10.2215/CJN.0000000000000161. Epub 2023 Mar 31. Clin J Am Soc Nephrol. 2023. PMID: 36999981 Free PMC article. Clinical Trial. - Kidney and Cardiovascular Effects of Canagliflozin According to Age and Sex: A Post Hoc Analysis of the CREDENCE Randomized Clinical Trial.
Yi TW, Smyth B, Di Tanna GL, Arnott C, Cardoza K, Kang A, Pollock C, Agarwal R, Bakris G, Charytan DM, de Zeeuw D, Heerspink HJL, Neal B, Wheeler DC, Cannon CP, Zhang H, Zinman B, Perkovic V, Levin A, Mahaffey KW, Jardine M; CREDENCE Trial Investigators. Yi TW, et al. Am J Kidney Dis. 2023 Jul;82(1):84-96.e1. doi: 10.1053/j.ajkd.2022.12.015. Epub 2023 Mar 7. Am J Kidney Dis. 2023. PMID: 36889425 Clinical Trial. - Canagliflozin for the Treatment of Diabetic Kidney Disease and Implications for Clinical Practice: A Narrative Review.
Kruger D, Valentine V. Kruger D, et al. Diabetes Ther. 2020 Jun;11(6):1237-1250. doi: 10.1007/s13300-020-00826-w. Epub 2020 May 13. Diabetes Ther. 2020. PMID: 32405876 Free PMC article. Review.
Cited by
- Sodium-glucose co-transporter protein 2 (SGLT2) inhibitors for people with chronic kidney disease and diabetes.
Natale P, Tunnicliffe DJ, Toyama T, Palmer SC, Saglimbene VM, Ruospo M, Gargano L, Stallone G, Gesualdo L, Strippoli GF. Natale P, et al. Cochrane Database Syst Rev. 2024 May 21;5(5):CD015588. doi: 10.1002/14651858.CD015588.pub2. Cochrane Database Syst Rev. 2024. PMID: 38770818 Review. - SGLT2 Inhibitors: Paradigm Shift from Diabetes Care to Metabolic Care-An Indian Perspective.
Kumar KMP, Unnikrishnan AG, Jariwala P, Mehta A, Chaturvedi R, Panchal S, Lakhani P, Acharya R, Dixit J. Kumar KMP, et al. Indian J Endocrinol Metab. 2024 Jan-Feb;28(1):11-18. doi: 10.4103/ijem.ijem_377_23. Epub 2024 Feb 26. Indian J Endocrinol Metab. 2024. PMID: 38533279 Free PMC article. Review. - Impact of metformin on cardiovascular and kidney outcome based on kidney function status in type 2 diabetic patients: a multicentric, retrospective cohort study.
Yi Y, Kwon EJ, Yun G, Park S, Jeong JC, Na KY, Chin HJ, Yoo S, Kim S, Oh TJ, Kim S, Jung CH, Lee H. Yi Y, et al. Sci Rep. 2024 Jan 24;14(1):2081. doi: 10.1038/s41598-024-52078-4. Sci Rep. 2024. PMID: 38267451 Free PMC article. - Impact of Canagliflozin on Kidney and Cardiovascular Outcomes by Type 2 Diabetes Duration: A Pooled Analysis of the CANVAS Program and CREDENCE Trials.
Tobe SW, Mavrakanas TA, Bajaj HS, Levin A, Tangri N, Slee A, Neuen BL, Perkovic V, Mahaffey KW, Rapattoni W, Ang FG. Tobe SW, et al. Diabetes Care. 2024 Mar 1;47(3):501-507. doi: 10.2337/dc23-1450. Diabetes Care. 2024. PMID: 38252809 Free PMC article. - 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2024.
American Diabetes Association Professional Practice Committee. American Diabetes Association Professional Practice Committee. Diabetes Care. 2024 Jan 1;47(Suppl 1):S219-S230. doi: 10.2337/dc24-S011. Diabetes Care. 2024. PMID: 38078574 Review.
References
- Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. - PubMed
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. - PMC - PubMed
- International Diabetes Federation . IDF Diabetes Atlas. ed 7. Brussels: International Diabetes Federation; 2015.
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. - PubMed