Hepatitis B virus x protein induces epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating long non-coding RNA - PubMed (original) (raw)
Hepatitis B virus x protein induces epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating long non-coding RNA
Yinji Jin et al. Virol J. 2017.
Erratum in
- Correction: Hepatitis B virus x protein induces epithelial-mesenchymal transition of hepatocellular carcinoma cells by regulating long non-coding RNA.
Jin Y, Wu D, Yang W, Weng M, Li Y, Wang X, Zhang X, Jin X, Wang T. Jin Y, et al. Virol J. 2026 Feb 9;23(1):36. doi: 10.1186/s12985-026-03083-8. Virol J. 2026. PMID: 41664161 Free PMC article. No abstract available.
Abstract
Background: It has been widely accepted that hepatitis B virus X protein (HBx) plays an important role in hepatocellular carcinoma (HCC). This study aimed to explore the function of long non-coding RNAs (lncRNAs) in the epithelial-mesenchymal transition (EMT) induced by HBx.
Methods: The association between HBx and EMT markers was detected using immunohistochemistry in HCC tissues. The effect of HBx on HCC EMT was assessed through morphological analysis, transwell assay, metastatic in vivo study and detection of EMT markers. LncRNA microarray was used to screen the differently expressed lncRNAs. Small interfering RNA and Western blot were used to analyse the function and mechanism of the locked lncRNA.
Results: HBx was negatively correlated with the epithelial marker E-cadherin but positively correlated with the mesenchymal marker vimentin in HCC tissues. HBx induced the mesenchymal phenotype and improved the metastatic ability of HCC cells. Meanwhile, HBx down-regulated E-cadherin, whereas it up-regulated vimentin. In HCC cells, HBx altered the expression of 2002 lncRNAs by more than 2-fold. One of them was ZEB2-AS1. Inhibition of ZEB2-AS1 can compensate for the EMT phenotype and reverse the expression of EMT markers regulated by HBx. Additionally, HBx affected the Wnt signalling pathway.
Conclusions: HBx promotes HCC cell metastasis by inducing EMT, which is at least partly mediated by lncRNAs.
Keywords: Epithelial-mesenchymal transition; Hepatitis B virus x protein; Hepatocellular carcinoma; Long non-coding RNA; Metastasis.
Conflict of interest statement
Ethics approval and consent to participate
All patients gave their informed consent prior to their inclusion in the study. This study was approved by the ethics committee of Harbin Medical University (reference numbers: HMUIRB20130089). This study was carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
The expression of HBx and EMT markers was detected by immuno- histochemistry on the HCC tissue microarray. The expression of E-cadherin was decreased but that of vimentin was increased in the HCC tissues with high levels of HBx compared to those with low levels of HBx (100×).The top right corner is the enlarged image (400×)
Fig. 2
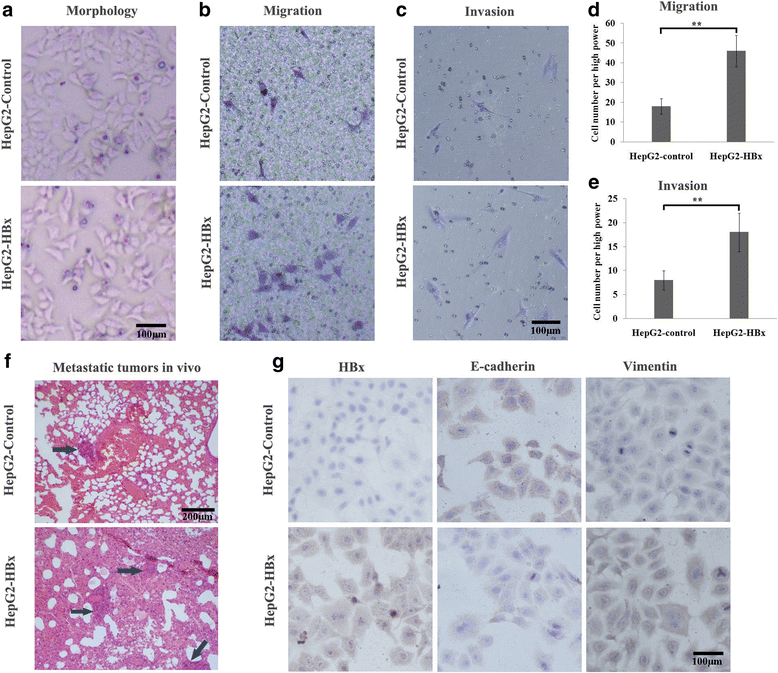
The effect of HBx on HCC EMT was detected by in vivo and in vitro experiments. a Morphology observation under the inverted microscope. HBx induced morphological alterations from the epithelial phenotype to the mesenchymal phenotype characterized by shuttle shape and scattered growth. b and c Transwell assays for migration and invasion. HBx promoted the metastatic ability and induced more cells to pass through the membrane. d and e The number of transmembrane cells are presented by column diagram. f Lung metastatic tumours in mice. The number of metastatic tumours was significantly higher in the HBx overexpressed HepG2 cells than the control group. g Immunocytochemistry assay for HBx, E-cadherin and vimentin. HBx overexpression in HepG2 cells resulted in decreased of E-cadherin and increased of vimentin. *p < 0.05, **p < 0.01
Fig. 3
ZEB2-AS1 was an important lncRNA that mediated HBx-induced EMT. a The level of ZEB2-AS1 was verified by a real-time PCR assay. The siRNA can effectively inhibit the level of ZEB2-AS1. b Transwell assay. Knockdown of ZEB2-AS1 inhibited the metastatic ability of HepG2 cells. c. The level of ZEB2-AS1 was verified by a real-time PCR assay. ZEB2-AS1 was up-regulated by 3.79-fold by HBx, but compromised by si-ZEB2-AS1 in HepG2-HBx. d and e The regulating effect of ZEB2-AS1 on EMT was assessed by transwell assay. HBx promoted the metastatic ability of HCC cells; however, siRNA treatment against ZEB2-AS1 rescued the phenotype in these cells. f EMT markers were detected by Western blot. HBx overexpression enhanced vimentin and ZEB2 expression, whereas it inhibited E-cadherin expression. However, knockdown ZEB2-AS1 reversed the above protein expression. *p < 0.05, **p < 0.01
Fig. 4
Analysis for microarray results. a GO enrichment analysis. All the altered mRNAs were significantly enriched in 41 GO terms. b KEGG pathway enrichment analysis. Five KEGG pathways were significantly enriched
References
- Nanashima A, et al. Clinicopathological characteristics of patients with hepatocellular carcinoma after hepatectomy: relationship with status of viral hepatitis. J SurgOncol. 2007;96(6):487–492. - PubMed
- Tanaka K, et al. Clinical characteristics and surgical outcome in hepatocellular carcinoma without hepatitis B virus surface antigen or hepatitis C virus antibody. Ann SurgOncol. 2007;14(3):1170–1181. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous