Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review - PubMed (original) (raw)
Review
Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review
Matthew J Page et al. Syst Rev. 2017.
Abstract
Background: The PRISMA Statement is a reporting guideline designed to improve transparency of systematic reviews (SRs) and meta-analyses. Seven extensions to the PRISMA Statement have been published to address the reporting of different types or aspects of SRs, and another eight are in development. We performed a scoping review to map the research that has been conducted to evaluate the uptake and impact of the PRISMA Statement and extensions. We also synthesised studies evaluating how well SRs published after the PRISMA Statement was disseminated adhere to its recommendations.
Methods: We searched for meta-research studies indexed in MEDLINE® from inception to 31 July 2017, which investigated some component of the PRISMA Statement or extensions (e.g. SR adherence to PRISMA, journal endorsement of PRISMA). One author screened all records and classified the types of evidence available in the studies. We pooled data on SR adherence to individual PRISMA items across all SRs in the included studies and across SRs published after 2009 (the year PRISMA was disseminated).
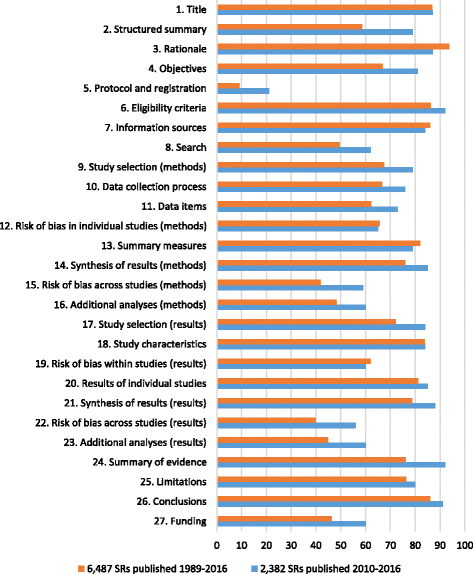
Results: We included 100 meta-research studies. The most common type of evidence available was data on SR adherence to the PRISMA Statement, which has been evaluated in 57 studies that have assessed 6487 SRs. The pooled results of these studies suggest that reporting of many items in the PRISMA Statement is suboptimal, even in the 2382 SRs published after 2009 (where nine items were adhered to by fewer than 67% of SRs). Few meta-research studies have evaluated the adherence of SRs to the PRISMA extensions or strategies to increase adherence to the PRISMA Statement and extensions.
Conclusions: Many studies have evaluated how well SRs adhere to the PRISMA Statement, and the pooled result of these suggest that reporting of many items is suboptimal. An update of the PRISMA Statement, along with a toolkit of strategies to help journals endorse and implement the updated guideline, may improve the transparency of SRs.
Keywords: Methodology; Quality; Reporting; Systematic reviews.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We have read the journal’s policy, and the authors of this manuscript have the following competing interests: DM is co-Editor in Chief, and MJP is an Associate Editor for Systematic Reviews, but neither had involvement in the peer review process or decision for publication. DM led the development of the PRISMA Statement. MJP and DM are leading the update of the PRISMA Statement. DM is an unpaid advisor to StatReviewer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
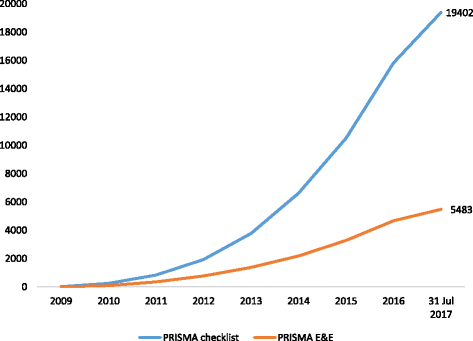
Fig. 1
Cumulative number of citations of the PRISMA Statement. Data obtained from Scopus® on 31 July 2017. E&E explanation and elaboration
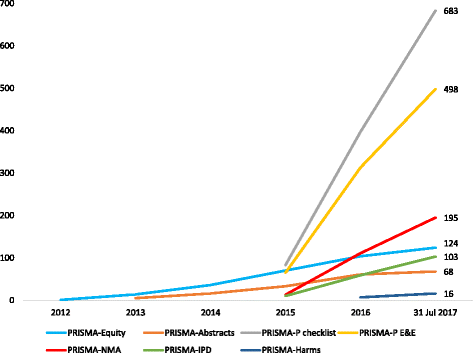
Fig. 2
Cumulative number of citations of PRISMA extensions published before 2017. Data obtained from Scopus® on 31 July 2017. E&E explanation and elaboration, IPD individual participant data, NMA network meta-analysis
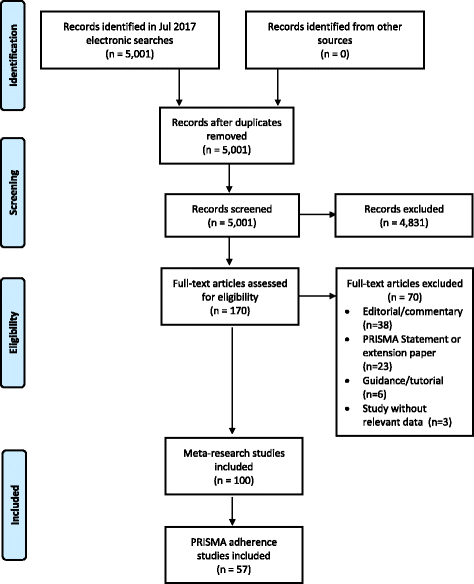
Fig. 3
Flow diagram of identification, screening and inclusion of studies
Fig. 4
Summary percentage across reports of SRs adhering to the PRISMA Statement
Similar articles
- Endorsement of PRISMA statement and quality of systematic reviews and meta-analyses published in nursing journals: a cross-sectional study.
Tam WW, Lo KK, Khalechelvam P. Tam WW, et al. BMJ Open. 2017 Feb 7;7(2):e013905. doi: 10.1136/bmjopen-2016-013905. BMJ Open. 2017. PMID: 28174224 Free PMC article. - Reporting Quality of Systematic Reviews and Meta-Analyses of Otorhinolaryngologic Articles Based on the PRISMA Statement.
Peters JP, Hooft L, Grolman W, Stegeman I. Peters JP, et al. PLoS One. 2015 Aug 28;10(8):e0136540. doi: 10.1371/journal.pone.0136540. eCollection 2015. PLoS One. 2015. PMID: 26317406 Free PMC article. Review. - Exploring reporting quality of systematic reviews and Meta-analyses on nursing interventions in patients with Alzheimer's disease before and after PRISMA introduction.
Sun X, Zhou X, Yu Y, Liu H. Sun X, et al. BMC Med Res Methodol. 2018 Nov 29;18(1):154. doi: 10.1186/s12874-018-0622-7. BMC Med Res Methodol. 2018. PMID: 30497417 Free PMC article. - Quality of reporting of systematic reviews and meta-analyses in emergency medicine based on the PRISMA statement.
Nawijn F, Ham WHW, Houwert RM, Groenwold RHH, Hietbrink F, Smeeing DPJ. Nawijn F, et al. BMC Emerg Med. 2019 Feb 11;19(1):19. doi: 10.1186/s12873-019-0233-6. BMC Emerg Med. 2019. PMID: 30744570 Free PMC article. - Adherence to the PRISMA statement and its association with risk of bias in systematic reviews published in rehabilitation journals: A meta-research study.
Innocenti T, Feller D, Giagio S, Salvioli S, Minnucci S, Brindisino F, Cosentino C, Piano L, Chiarotto A, Ostelo R. Innocenti T, et al. Braz J Phys Ther. 2022 Sep-Oct;26(5):100450. doi: 10.1016/j.bjpt.2022.100450. Epub 2022 Oct 14. Braz J Phys Ther. 2022. PMID: 36270163 Free PMC article. Review.
Cited by
- Global Overview of Suicidal Behavior and Risk Factors among General Population during the COVID-19 Pandemic: A Systematic Review and a Meta-Regression.
Mudiyanselage SPK, Tsai YT, Dilhani MS, Tsai YJ, Yang YH, Lu ZT, Ko NY. Mudiyanselage SPK, et al. Psychiatr Q. 2024 Oct 31. doi: 10.1007/s11126-024-10096-5. Online ahead of print. Psychiatr Q. 2024. PMID: 39480625 Review. - Assessment of the Mental Health of Police Officers: A Systematic Review of Specific Instruments.
Teles DO, Oliveira RA, Parnaíba ALO, Rios MA, Machado MB, Aquino PS, Menezes PR, Ribeiro SG, Soares PRAL, Biazus Dalcin C, Pinheiro AKB. Teles DO, et al. Int J Environ Res Public Health. 2024 Sep 28;21(10):1300. doi: 10.3390/ijerph21101300. Int J Environ Res Public Health. 2024. PMID: 39457273 Free PMC article. Review. - Exploring transparent reporting and data availability in systematic reviews to identify subgroup evidence: imaging for suspected hepatocellular carcinoma in the non-cirrhotic liver.
Oerbekke MS, de Man RA, van Vilsteren FGI, Nijkamp MW, Tjwa E, Gaasterland CMW, van der Laan MJ, Hooft L. Oerbekke MS, et al. Orphanet J Rare Dis. 2024 Oct 2;19(1):364. doi: 10.1186/s13023-024-03356-x. Orphanet J Rare Dis. 2024. PMID: 39358755 Free PMC article. - Efficacy of Corticosteroids for Sore Throat Management in Adults: A Systematic Review and Meta-Analysis of Randomized Trials.
Alqahtani S, Alsharidi TRI, Alelaiwi MA, Albelowi LM, Alserhani AS, Alhosan ZA, Alhazmi RM, Alaithan MA, Almutairi AO, Alqahtani L, Alhammad AS, Aljahdali HF, Althomali F, Basalamah MA. Alqahtani S, et al. Cureus. 2024 Aug 25;16(8):e67740. doi: 10.7759/cureus.67740. eCollection 2024 Aug. Cureus. 2024. PMID: 39318911 Free PMC article. Review. - Smoking cessation and risk of metabolic syndrome: A meta-analysis.
Kim HJ, Cho YJ. Kim HJ, et al. Medicine (Baltimore). 2024 May 31;103(22):e38328. doi: 10.1097/MD.0000000000038328. Medicine (Baltimore). 2024. PMID: 39259087 Free PMC article.
References
- Agoritsas T, Vandvik PO, Neumann I, Rochwerg B, Jaeschke R, Hayward R, et al. Chapter 5: finding current best evidence. In: Guyatt G, Rennie D, Meade MO, Cook DJ, et al., editors. Users’ guides to the medical literature: a manual for evidence-based clinical practice. 3. New York: McGraw-Hill; 2015. pp. 29–50.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials