Srf controls satellite cell fusion through the maintenance of actin architecture - PubMed (original) (raw)
. 2018 Feb 5;217(2):685-700.
doi: 10.1083/jcb.201705130. Epub 2017 Dec 21.
Voahangy Randrianarison-Huetz 1 2 3, Aikaterini Papaefthymiou 1 2 3, Gaëlle Herledan 1 2 3, Chiara Noviello 1 2 3, Ulduz Faradova 1 2 3, Alessandra Pincini 1 2 3, Emilie Schol 1 2 3, Jean François Decaux 5, Pascal Maire 1 2 3, Stéphane Vassilopoulos 6, Athanassia Sotiropoulos 7 2 3
Affiliations
- PMID: 29269426
- PMCID: PMC5800804
- DOI: 10.1083/jcb.201705130
Srf controls satellite cell fusion through the maintenance of actin architecture
Voahangy Randrianarison-Huetz et al. J Cell Biol. 2018.
Abstract
Satellite cells (SCs) are adult muscle stem cells that are mobilized when muscle homeostasis is perturbed. Here, we show that serum response factor (Srf) is needed for optimal SC-mediated hypertrophic growth. We identified Srf as a master regulator of SC fusion required in both fusion partners, whereas it was dispensable for SC proliferation and differentiation. We show that SC-specific Srf deletion leads to impaired actin cytoskeleton and report the existence of finger-like actin-based protrusions at fusion sites in vertebrates that were notoriously absent in fusion-defective myoblasts lacking Srf. Restoration of a polymerized actin network by overexpression of an α-actin isoform in Srf mutant SCs rescued their fusion with a control cell in vitro and in vivo and reestablished overload-induced muscle growth. These findings demonstrate the importance of Srf in controlling the organization of actin cytoskeleton and actin-based protrusions for myoblast fusion in mammals and its requirement to achieve efficient hypertrophic myofiber growth.
© 2018 Randrianarison-Huetz et al.
Figures
Figure 1.
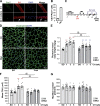
Srf loss in SCs results in CH deficiency in plantaris muscle. (A) Immunostaining for Pax7 (green) and Srf (red) on single fibers fixed immediately after isolation (0 h) or maintained in culture for 24 h. White arrows indicate SC expressing both Srf and Pax7. (B) Proportion of SCs displaying Srf expression (Pax7+Srf+; n = 3). (C) Srf mutant mice were injected with TMX 1 wk before CH procedure and after CH. Plantaris muscles were isolated 1, 3, and 5 wk after surgery. (D) Plantaris muscle sections immunostained for dystrophin (green) and nuclear staining with DAPI for control and Srf Mutant mice before (SO) and after 3 wk of CH. (E) Ratio of plantaris mass (milligrams) to body weight (grams) before (SO) and after 1, 3, and 5 wk of CH in control and Mutant mice (n = 10–16 muscles from n = 6–9 mice). (F) Mean CSA (square micrometers) before (SO) and after 1, 3, and 5 wk of CH in control and mutant mice (n = 6–15 muscles from n = 5–9 mice). (G) Mean myofiber number before (SO) and after 1, 3, and 5 wk of CH in control and mutant mice (n = 8–14 muscles from n = 6–9 mice). Data are mean ± SEM. **, P < 0.01 versus SO; §§, P < 0.01.
Figure 2.
Srf loss within SCs does not affect their proliferation but impairs their motility. (A) control and Srf mutant plantaris muscle section immunostained for Pax7 (green), laminin (magenta), and nuclear staining with DAPI 1 wk after CH. Yellow arrows indicate Pax7-expressing SCs. (B) Number of Pax7+ cells per myofiber in control and mutant plantaris muscle sections before (SO) and after 1, 3, and 5 wk of CH (n = 7–12 muscles from n = 4–7 mice). (C) Normalized percentage of EdU+ cells in control and mutant FACS-sorted SCs cultured in rich medium for 5 d (n = 8–5 mice). (D) Percentage of PH3+ cells in control and mutant FACS-sorted SCs cultured in rich medium for 5 d (n = 3–4 mice). (E) Mean velocity (micrometers per minute) of control and mutant MBs determined by time-lapse videomicroscopy (one representative experiment). Data are means ± SEM. *, P < 0.05; **, P < 0.01.
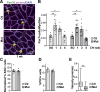
Figure 3.
Srf loss does not affect myogenic differentiation of SCs. (A) Number of MyoG+ cells per myofiber in control and Srf mutant plantaris muscle sections before (SO) and after 1 wk of CH (n = 6–10 muscles from n = 4–6 mice). (B) Percentage of MyoD+ cells in control and mutant FACS-sorted SCs cultured in rich medium (D0, MBs) or 1 d after differentiation induction (D1; n = 4). (C) Percentage of MyoG+ in control and mutant FACS-sorted SCs cultured in rich medium (D0) or 1 (D1) and 3 (D3) days after differentiation induction (n = 4–5). (D) Immunostaining for MyHC, nuclear staining with DAPI, and F-actin staining with phalloidin on control and mutant cells 3 d after differentiation induction. (E) Percentage of nuclei in MyHC+ cells in control and mutant cells 3 d after differentiation induction (n = 5–6). Data are mean ± SEM. **, P < 0.01.
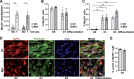
Figure 4.
Srf controls SC fusion. (A) Number of nuclei (DAPI) within the dystrophin+ sarcolemma per myofiber before (SO) and after 1, 3, and 5 wk of CH in control and Srf mutant plantaris muscles (n = 6–11 muscles from n = 4–6 mice). Data are mean ± SEM. *, P < 0.05 versus SO; **, P < 0.01 versus SO; §§, P < 0.01. (B) Phase-contrast representative pictures of FACS-sorted control and mutant SCs cultured in rich medium (D0) or 1 (D1) and 3 (D3) days after differentiation induction. (C) Proportion of nuclei within multinucleated cells (fusion index) in control and mutant cells 3 d after differentiation (n = 5–6). (D) Mean number of nuclei per MyHC+ cell in control and mutant cells induced to differentiate for 3 d (n = 4–5). (E) Control and mutant MB were labeled with Orange Cell Tracker and mixed with control or mutant MBs labeled with Deep Red Cell Tracker. After 48 h of co-culture in differentiation medium, the percentage of dual-labeled MTs per total number of nuclei was scored (n = 3–6). (F) MT control were labeled with Orange Cell Tracker and mixed with MB control or MB mutant labeled with Deep Red Cell Tracker. After 48 h of co-culture, the percentage of dual-labeled MTs per total number of cells was scored (n = 4–5). For C–F, data are mean ± SEM. *, P < 0.05; **, P < 0.01.
Figure 5.
Srf controls the expression of actin genes and genes implicated in actin cytoskeleton regulation. (A) Venn diagram showing the intersections between genes differentially regulated by Srf (P < 0.05) in MBs (D0), myocytes at onset of differentiation (D1), and differentiated cells (D3). In red is indicated the number of genes (144) that are modulated by Srf independently of the differentiation state. (B) Top five canonical pathways identified by gene ontology analysis using Ingenuity of the 144 common genes whose expression is Srf dependent. (C) Analysis of α-skeletal actin (Acta1), α-cardiac actin (Actc1), smooth muscle actin (Acta2), γ-actin (Actg), and β-actin (Actb) mRNA expression by qRT-PCR in FACS-sorted control and Srf mutant SCs cultured in rich medium (D0) or 1 (D1) and 3 (D3) days after differentiation. Data were normalized by Hmbs expression and relative to D0 (n = 3). (D) Analysis of Abra, Cnn2, Fermt2, FlnA, Tgfb1i1, and Wdr1 mRNA expression by qRT-PCR in FACS-sorted control and mutant SCs cultured in rich medium (D0) or 1 (D1) and 3 (D3) days after differentiation. Data were normalized by Hmbs expression and relative to D0 (n = 3). Data are mean ± SEM. *, P < 0.05; **, P < 0.01.
Figure 6.
α-Actin overexpression restores the impaired F-actin content of _Srf_-deleted MBs. (A) Staining for F-actin (phalloidin) and nuclei (DAPI) on control, _Srf-_deleted (Mut), and _Srf_-deleted MBs overexpressing α-actin (Mut/Act+). (B) Quantification of F-actin by measuring the total phalloidin fluorescence intensity per cell (ImageJ) in control, mutant, and Mut/Act+ MBs (one representative experiment). (C) Representative immunoblot showing total actin (pan-Actin) in control, mutant, and Mut/Act+ MBs. Tubulin was used as a loading control. (D) Quantification of the pan-actin/tubulin ratio from immunoblots (n = 5–9). (E) Representative immunoblot showing actin in the insoluble (F) versus soluble (G) fractions in control, mutant, and Mut/Act+ MBs. (F) Quantification of the F-/G-actin ratio from immunoblots (n = 6–8). (G) Representative confocal projections of _z_-sections of F-actin staining (phalloidin) taken from the adherent (ventral) cell bottom and middle and proceeding up to the media-facing top of control, mutant, and Mut/Act+ MBs. (H) Survey view of the cytoplasmic surface of the plasma membrane from unroofed control MBs. (H′) Higher-magnification view from H. (H″) Higher-magnification view corresponding to the boxed regions in H′. (I) Survey view of the cytoplasmic surface of the plasma membrane from unroofed mutant MBs. (I′) Higher-magnification view from I. (I″) Higher-magnification view corresponding to the boxed regions in I′. (J) Survey view of the cytoplasmic surface of the plasma membrane from unroofed Mut/Act+ MBs. (J′) Higher-magnification view from J. (J″) Higher-magnification view corresponding to the boxed region in J′. Data are mean ± SEM. *, P < 0.05; **, P < 0.01.
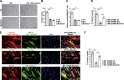
Figure 7.
Formation of finger-like actin-based protrusions at the site of fusion. (A) Survey view of the cytoplasmic surface of the plasma membrane from control muscle cells differentiated for 24 h. Cell 1: Pseudocolored in purple forms finger-like protrusions that are traceable below the acceptor cell 2. (A′) Higher-magnification view from A of the finger-like protrusions denoted with yellow arrows. (B) Higher-magnification view of the finger-like protrusions from a double unroofed cell–cell contact. White arrows, actin cables from attacking cell 1; yellow arrows, finger-like protrusions traceable below cell 2; *, branched actin filaments. (C) Survey view of the cytoplasmic surface of the plasma membrane from two unroofed control muscle cells differentiated for 24 h that have successfully fused. Cell 1 presenting the protrusions is pseudocolored in purple. White arrowheads denote the fusion site. (C′) Higher-magnification view of the fusion site from boxed region in C. Yellow arrows denote finger-like protrusions below the fusion site. (D) Survey view of the cytoplasmic surface from mutant muscle cells differentiated for 24 h. Note clusters of MBs (each MB is a different pseudocolor) unable to fuse. (D′ and D″) Higher-magnification views of cell contact sites from the boxed regions in C. (E) Survey view of the cytoplasmic surface from Mut/Act+ cells differentiated for 24 h. (E′) Higher-magnification view from E.
Figure 8.
α-Actin overexpression in MBs lacking Srf restores heterotypic fusion. (A) Phase-contrast representative pictures of FACS-sorted control and Mut/Act+ SCs cultured in rich medium (D0) or 3 d after differentiation induction (D3). (B) Proportion of nuclei within multinucleated cells (fusion index) in control, mutant, and Mut/Act+ cells 3 d after differentiation (n = 4–7). (C) Mean number of nuclei per MyHC+ cell in control, mutant, and Mut/Act+ cells induced to differentiate for 3 d (n = 4–5). (D) MB control, MB mutant, or MB Mut/Act+ were labeled with Green Cell Tracker and mixed with control MBs labeled with Orange Cell Tracker. After 48 h of co-culture in differentiation medium, the percentage of dual-labeled MTs per total number of nuclei was scored (n = 4–7). (E) MT control were labeled with Cell Tracker 1 (Orange; visualized in red) and mixed with MB control, MB mutant, or MB Mut/Act+ labeled with CellTracker 2 (Deep Red; visualized in green). After 48 h of co-culture, MTs were analyzed for dual labeling (indicated by arrow). (F) MT control were labeled with Orange Cell Tracker and mixed with MB control, MB mutant, or MB Mut/Act+ labeled with Deep Red Cell Tracker. The percentage of dual-labeled cells per total number of cells was scored (n = 4–5). Data are mean ± SEM. **, P < 0.01.
Figure 9.
α-Actin overexpression in Srf-deleted SCs rescues fusion and hypertrophic growth upon overload. (A) TA muscle sections immunostained for dystrophin (green) and nuclear staining with DAPI for control, mutant, and Mut/Act+ mice untreated (No CTX) and 30 d after CTX-induced muscle injury (30D after CTX). (B) Mean CSA (square micrometers) of TA muscles untreated (0) and 30 d after CTX-induced injury in control, mutant, and Mut/Act+ mice (n = 5–9 muscles from n = 4–7 mice). (C) Number of nuclei (DAPI) within the dystrophin+ sarcolemma per myofiber in untreated (0) and 30 d after CTX-induced injury of control, mutant, and Mut/Act+ TA muscles (n = 5–11 muscles from n = 4–7 mice). (D) Ratio of plantaris mass (milligrams) to body weight (grams) before (SO) and after 3 and 5 wk of CH in control, mutant, and Mut/Act+ mice (n = 5–16 muscles from n = 4–9 mice). (E) Mean CSA (square micrometers) of plantaris before (SO) and after 3 and 5 wk of CH in control, mutant, and Mut/Act+ mice (n = 5–15 muscles from n = 4–9 mice). (F) Number of nuclei (DAPI) within the dystrophin+ sarcolemma per myofiber before (SO) and after 3 and 5 wk of CH in control, mutant, and Mut/Act+ plantaris muscles (n = 6–11 muscles from n = 4–6 mice). Data are mean ± SEM. *, P < 0.05; **, P < 0.01.
Similar articles
- Srf-dependent paracrine signals produced by myofibers control satellite cell-mediated skeletal muscle hypertrophy.
Guerci A, Lahoute C, Hébrard S, Collard L, Graindorge D, Favier M, Cagnard N, Batonnet-Pichon S, Précigout G, Garcia L, Tuil D, Daegelen D, Sotiropoulos A. Guerci A, et al. Cell Metab. 2012 Jan 4;15(1):25-37. doi: 10.1016/j.cmet.2011.12.001. Cell Metab. 2012. PMID: 22225874 - Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells.
Schratt G, Philippar U, Berger J, Schwarz H, Heidenreich O, Nordheim A. Schratt G, et al. J Cell Biol. 2002 Feb 18;156(4):737-50. doi: 10.1083/jcb.200106008. Epub 2002 Feb 11. J Cell Biol. 2002. PMID: 11839767 Free PMC article. - RhoA Is a Crucial Regulator of Myoblast Fusion.
Noviello C, Kobon K, Randrianarison-Huetz V, Maire P, Pietri-Rouxel F, Falcone S, Sotiropoulos A. Noviello C, et al. Cells. 2023 Nov 21;12(23):2673. doi: 10.3390/cells12232673. Cells. 2023. PMID: 38067102 Free PMC article. - Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus.
Miano JM, Long X, Fujiwara K. Miano JM, et al. Am J Physiol Cell Physiol. 2007 Jan;292(1):C70-81. doi: 10.1152/ajpcell.00386.2006. Epub 2006 Aug 23. Am J Physiol Cell Physiol. 2007. PMID: 16928770 Review. - Actin-mediated gene expression in neurons: the MRTF-SRF connection.
Knöll B. Knöll B. Biol Chem. 2010 Jun;391(6):591-7. doi: 10.1515/BC.2010.061. Biol Chem. 2010. PMID: 20370316 Review.
Cited by
- Molecular Regulation of Skeletal Muscle Growth and Organelle Biosynthesis: Practical Recommendations for Exercise Training.
Solsona R, Pavlin L, Bernardi H, Sanchez AM. Solsona R, et al. Int J Mol Sci. 2021 Mar 8;22(5):2741. doi: 10.3390/ijms22052741. Int J Mol Sci. 2021. PMID: 33800501 Free PMC article. Review. - Dynamic remodeling of septin structures fine-tunes myogenic differentiation.
Ugorets V, Mendez PL, Zagrebin D, Russo G, Kerkhoff Y, Kotsaris G, Jatzlau J, Stricker S, Knaus P. Ugorets V, et al. iScience. 2024 Jul 31;27(9):110630. doi: 10.1016/j.isci.2024.110630. eCollection 2024 Sep 20. iScience. 2024. PMID: 39246450 Free PMC article. - Lineage tracing of newly accrued nuclei in skeletal myofibers uncovers distinct transcripts and interplay between nuclear populations.
Sun C, Swoboda CO, Petrany MJ, Parameswaran S, VonHandorf A, Weirauch MT, Lepper C, Millay DP. Sun C, et al. bioRxiv [Preprint]. 2023 Aug 25:2023.08.24.554609. doi: 10.1101/2023.08.24.554609. bioRxiv. 2023. PMID: 37662191 Free PMC article. Updated. Preprint. - Myonuclear Domain Flexibility Challenges Rigid Assumptions on Satellite Cell Contribution to Skeletal Muscle Fiber Hypertrophy.
Murach KA, Englund DA, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Murach KA, et al. Front Physiol. 2018 May 29;9:635. doi: 10.3389/fphys.2018.00635. eCollection 2018. Front Physiol. 2018. PMID: 29896117 Free PMC article. - IL-4 Signaling Promotes Myoblast Differentiation and Fusion by Enhancing the Expression of MyoD, Myogenin, and Myomerger.
Kurosaka M, Hung YL, Machida S, Kohda K. Kurosaka M, et al. Cells. 2023 Apr 29;12(9):1284. doi: 10.3390/cells12091284. Cells. 2023. PMID: 37174683 Free PMC article.
References
- Böttcher R.T., Veelders M., Rombaut P., Faix J., Theodosiou M., Stradal T.E., Rottner K., Zent R., Herzog F., and Fässler R.. 2017. Kindlin-2 recruits paxillin and Arp2/3 to promote membrane protrusions during initial cell spreading. J. Cell Biol. 216:3785–3798. 10.1083/jcb.201701176 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous