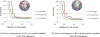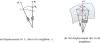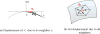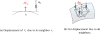Interactive Prostate Shape Reconstruction from 3D TRUS Images - PubMed (original) (raw)
Interactive Prostate Shape Reconstruction from 3D TRUS Images
Tomotake Furuhata et al. J Comput Des Eng. 2014 Oct.
Abstract
This paper presents a two-step, semi-automated method for reconstructing a three-dimensional (3D) shape of the prostate from a 3D transrectal ultrasound (TRUS) image. While the method has been developed for prostate ultrasound imaging, it can potentially be applicable to any other organ of the body and other imaging modalities. The proposed method takes as input a 3D TRUS image and generates a watertight 3D surface model of the prostate. In the first step, the system lets the user visualize and navigate through the input volumetric image by displaying cross sectional views oriented in arbitrary directions. The user then draws partial/full contours on selected cross sectional views. In the second step, the method automatically generates a watertight 3D surface of the prostate by fitting a deformable spherical template to the set of user-specified contours. Since the method allows the user to select the best cross-sectional directions and draw only clearly recognizable partial or full contours, the user can avoid time-consuming and inaccurate guesswork on where prostate contours are located. By avoiding the usage of noisy, incomprehensible portions of the TRUS image, the proposed method yields more accurate prostate shapes than conventional methods that demand complete cross-sectional contours selected manually, or automatically using an image processing tool. Our experiments confirmed that a 3D watertight surface of the prostate can be generated within five minutes even from a volumetric image with a high level of speckles and shadow noises.
Keywords: Image Processing; Prostate; Shape Reconstruction; TRUS; Ultrasound.
Figures
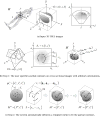
Figure 1
Typical TRUS images – part of an image is often not comprehensible due to speckle and shadow noises.
Figure 2
A prostate 3D geometry reconstructed from a set of manually selected full contours is not smooth and may not be watertight.
Figure 3
Proposed two-step approach to generating the 3D surface model of a prostate from a 3D TRUS image
Figure 4
Developed GUI for Step 1 cross-sectional image navigation, adjustment of intensity and contrast, and specification of partial/full contours.
Figure 5
Template polygonal models of the prostate
Figure 6
Error convergence with tri-mesh and quad-mesh templates. A quad-mesh template yields 30–40% smaller error compared with a tri-mesh template having a similar number of mesh elements.
Figure 7
3D Error comparison with various smoothing schemes.
Figure 8
V-Spring uses a spring force that acts in the normal direction to minimize curvature variation.
Figure 9
V-Spring uses a spring force that acts in the tangential direction to optimize vertex distribution.
Figure 10
V-Spring uses spring forces for fitting, or constraining, the final surface to approximate the set of partial contours.
Figure 11
Manually specified contours deviate approximately 1 mm on average with a maximum deviation of 2.5–3.0 mm, if the user is asked to plot a complete contour.
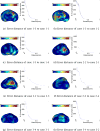
Figure 12
Error convergence with different types of input contours.
Figure 13
Shape convergence with multiple, randomly selected partial contours using Scheme 3
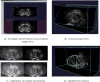
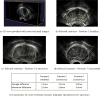
Figure 14
Case Study 3: the entire shape reconstruction process yields a smooth, realistic, and water-tight 3D shape of the prostate.
Figure 15
Examples of 3D prostate shapes created by the proposed shape reconstruction method.
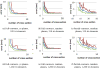
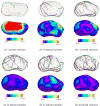
Figure 16
The error distances map and histogram of a process to the other process for each case
Figure 16
The error distances map and histogram of a process to the other process for each case
Similar articles
- Rotationally resliced 3D prostate TRUS segmentation using convex optimization with shape priors.
Qiu W, Yuan J, Ukwatta E, Fenster A. Qiu W, et al. Med Phys. 2015 Feb;42(2):877-91. doi: 10.1118/1.4906129. Med Phys. 2015. PMID: 25652500 - Toward a 3D transrectal ultrasound system for verification of needle placement during high-dose-rate interstitial gynecologic brachytherapy.
Rodgers JR, Surry K, Leung E, D'Souza D, Fenster A. Rodgers JR, et al. Med Phys. 2017 May;44(5):1899-1911. doi: 10.1002/mp.12221. Epub 2017 Apr 20. Med Phys. 2017. PMID: 28295403 - 2D-3D rigid registration to compensate for prostate motion during 3D TRUS-guided biopsy.
De Silva T, Fenster A, Cool DW, Gardi L, Romagnoli C, Samarabandu J, Ward AD. De Silva T, et al. Med Phys. 2013 Feb;40(2):022904. doi: 10.1118/1.4773873. Med Phys. 2013. PMID: 23387775 - Three-dimensional prostate segmentation using level set with shape constraint based on rotational slices for 3D end-firing TRUS guided biopsy.
Qiu W, Yuan J, Ukwatta E, Tessier D, Fenster A. Qiu W, et al. Med Phys. 2013 Jul;40(7):072903. doi: 10.1118/1.4810968. Med Phys. 2013. PMID: 23822454 - Clinical evaluation of an MRI-to-ultrasound deformable image registration algorithm for prostate brachytherapy.
Shaaer A, Davidson M, Semple M, Nicolae A, Mendez LC, Chung H, Loblaw A, Tseng CL, Morton G, Ravi A. Shaaer A, et al. Brachytherapy. 2019 Jan-Feb;18(1):95-102. doi: 10.1016/j.brachy.2018.08.006. Epub 2018 Oct 2. Brachytherapy. 2019. PMID: 30287271 Review.
Cited by
- Defeating Cancers' Adaptive Defensive Strategies Using Thermal Therapies: Examining Cancer's Therapeutic Resistance, Ablative, and Computational Modeling Strategies as a means for Improving Therapeutic Outcome.
Baust JM, Rabin Y, Polascik TJ, Santucci KL, Snyder KK, Van Buskirk RG, Baust JG. Baust JM, et al. Technol Cancer Res Treat. 2018 Jan 1;17:1533033818762207. doi: 10.1177/1533033818762207. Technol Cancer Res Treat. 2018. PMID: 29566612 Free PMC article. Review. - The role of exposure time in computerized training of prostate cryosurgery: performance comparison of surgical residents with engineering students.
Joshi P, Sehrawat A, Rabin Y. Joshi P, et al. Int J Comput Assist Radiol Surg. 2018 Apr;13(4):541-549. doi: 10.1007/s11548-017-1700-8. Epub 2018 Feb 2. Int J Comput Assist Radiol Surg. 2018. PMID: 29396685 - Computerized Planning of Prostate Cryosurgery and Shape Considerations.
Joshi P, Sehrawat A, Rabin Y. Joshi P, et al. Technol Cancer Res Treat. 2017 Dec;16(6):1272-1283. doi: 10.1177/1533034617716041. Epub 2017 Jul 21. Technol Cancer Res Treat. 2017. PMID: 28731368 Free PMC article. - A Computerized Tutor Prototype for Prostate Cryotherapy: Key Building Blocks and System Evaluation.
Rabin Y, Shimada K, Joshi P, Sehrawat A, Keelan R, Wilfong DM, McCormick JT. Rabin Y, et al. Proc SPIE Int Soc Opt Eng. 2017 Jan 28;10066:100660Z. doi: 10.1117/12.2257151. Epub 2017 Feb 22. Proc SPIE Int Soc Opt Eng. 2017. PMID: 28717259 Free PMC article. - GPU-Based Simulation of Ultrasound Imaging Artifacts for Cryosurgery Training.
Keelan R, Shimada K, Rabin Y. Keelan R, et al. Technol Cancer Res Treat. 2017 Feb;16(1):5-14. doi: 10.1177/1533034615623062. Epub 2016 Jun 23. Technol Cancer Res Treat. 2017. PMID: 26818026 Free PMC article.
References
- Developing a computerized training tool for cryosurgery. http://projectreporter.nih.gov/project_info_description.cfm?projectnumbe....
- Fenster A, Downey D, Cardinal N. Three Dimensional Ultrasound Imaging. Physics in Medicine and Biology. 2001;46(5):67–99. - PubMed
- Tong S, Downey D, Cardinal N, Fenster A. A three-dimensional ultrasound prostate imaging system. Ultrasound Med, Biol. 1996;22:735–746. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources