STING-Dependent Signaling Underlies IL-10 Controlled Inflammatory Colitis - PubMed (original) (raw)
STING-Dependent Signaling Underlies IL-10 Controlled Inflammatory Colitis
Jeonghyun Ahn et al. Cell Rep. 2017.
Abstract
Intestinal immune homeostasis is preserved by commensal bacteria interacting with the host to generate a balanced array of cytokines that are essential for wound repair and for combatting infection. Inflammatory bowel disease (IBD), which can lead to colitis-associated cancer (CAC), is thought to involve chronic microbial irritation following a breach of the mucosal intestinal epithelium. However, the innate immune pathways responsible for regulating these inflammatory processes remain to be fully clarified. Here, we show that commensal bacteria influence STING signaling predominantly in mononuclear phagocytes to produce both pro-inflammatory cytokines as well as anti-inflammatory IL-10. Enterocolitis, manifested through loss of IL-10, was completely abrogated in the absence of STING. Intestinal inflammation was less severe in the absence of cGAS, possibly suggesting a role for cyclic dinucleotides (CDNs) indirectly regulating STING signaling. Our data shed insight into the causes of inflammation and provide a potential therapeutic target for prevention of IBD.
Keywords: CAC; IBD; IL-10; STING; colitis-associated cancer; inflammatory bowel disease.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
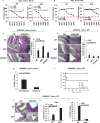
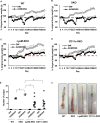
Figure 1. Commensal bacteria-host interactions influence colonic polyp formation
Body weight assessment of B6 background of Wild-type (WT) and Sting Knockout (SKO) mice received 5% of Dextran sodium sulfate (DSS) in drinking water for 7 days in (A) helico+ barrier room or helico− SPF room (B). The data are representative of at least two independent experiments. (C) Representative photographs of Hematoxylin and eosin staining (H&E) of colon tissues and the number of polyps in colon tissue of WT and SKO mice either AOM/DSS-treated or normal water treated in the helico+ barrier room. (D) The number of polyps in AOM/DSS-treated SKO mice either antibiotics treated or not. An antibiotic cocktail was administered in the drinking water of a separate cohort of mice for 1 month prior to AOM/DSS treat. (E) Representative photographs of H&E staining and the number of polyps in colon tissue of WT and SKO mice either AOM/DSS-treated or normal water treated in the helico− SPF room. (F) Mortality rates of AOM/DSS-treated WT, SKO, and cGASKO mice in helico− SPF room. (G) Representative photographs of H&E staining and (H) Inflammation score (0: Normal to 3: most severe) in either AOM/DSS-treated or normal water treated in helico− SPF room for 7 days. (I) quantitative PCR (qPCR) analysis of IL10 expression in colon from the mice same as (H). Error bars indicated s. d. Statistical analysis was performed using Student’s t-test. *; p ≤ 0.05
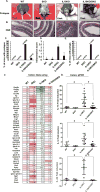
Figure 2. IL10 suppresses STING-induced inflammatory colitis and CAC
(A) Representative photographs of rectal prolapse, (B) representative photographs of H&E staining, (C) % of mice with prolapse (D) inflammation score, and (E) the number of polyps in 10~19 weeks old WT (n=10), SKO (n=15), IL10KO (n=26) and IL10KO/SKO mice (n=19). (F) Gene array analysis of colon tissue from 8 weeks old age of WT, SKO, IL10KO and IL10KO/SKO mice (n=5). Fold changes were estimated by WT mice and the highest variable genes are shown. Pseudo-colors indicate transcript levels equal to below (red) or above (green). (G) qPCR of IL1β, IL12, and IL22 mRNA level in in each genotype of colon tissue. All data are the mean of at least 7 mice. Error bars indicated s. d. Statistical analysis was performed using Student’s t-test. *; p ≤ 0.05
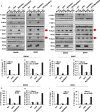
Figure 3. STING-signaling does not require the adaptor MyD88
(A, E) Gene array analysis from total RNA purified in WT, SKO and MyD88KO mouse embryonic fibroblasts (MEFs) treated with 3ug/ml of ISD (A) or 1 ug/ml of LPS (E) for 6 hours. Fold changes were estimated by WT mice and the highest variable genes are shown. Pseudo-colors indicate transcript levels equal to below (red) and above (green). qPCR analysis of IFNβ mRNA level in MEFs (B), Bone marrow derived macrophages (BMDM) (C) and Bone marrow derived dendritic cells (BMDC) (D) treated same as (A). qPCR analysis of IL1β mRNA level in MEFs (F), BMDM (G) and BMDC (H) treated same as (E).
Figure 4. STING-signaling drives pro-inflammatory, MyD88-dependent gene induction
(A, B) Immunoblot analysis to determine the levels of pTBK1, pIRF3, pStat1, pStat3, and STING in BMDM or BMDC treated with 3ug/ml of ISD (A) or 3ug/ml of LPS (B) for 6 hours. qPCR analysis of IL1β mRNA level in BMDM (C, D) or BMDC (E, F) treated with 3ug/ml of ISD (C, E) or 3ug/ml of c-di-GMP (E, F) for 6 hours. qPCR analysis of IL18 mRNA level in BMDM (G, H) or BMDC (I, J) treated with 3ug/ml of ISD (G, I) or 3ug/ml of c-di-GMP (H, J) for 6 hours. Data are representative of at least 2 independent experiments. Error bars indicated s. d.
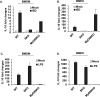
Figure 5. IL10 can be modulated by STING signaling
qPCR analysis of IL10 mRNA level in BMDM (A, C) or BMDC (B, D) treated with 3ug/ml of ISD (A, B) or 1ug/ml of LPS (C, D) for 6 hours. Data are representative of at least 2 independent experiments. Error bars indicated s. d.
Figure 6. Monocytes are predominantly responsible for STING-mediated pro-inflammatory responses
Body weight assessment in WT (A), SKO (B), LysM-SKO (C) and CD11c-SKO (D) mice treated with AOM/DSS- or normal water as control. LysM-SKO for AOM/DSS (n=10), LysM-SKO for DW control (n=6), CD11c-SKO for AOM/DSS (n=9), CD11c-SKO for DW control (n=5), WT for AOM/DSS (n=4), WT for DW control (n=3), SKO for AOM/DSS (n=4), and SKO mice for DW control (n=5). (E) Number of polys (F) and representative photographs of polyps in colon. All data are the mean of at least 3 mice. Error bars indicated s. d. Statistical analysis was performed using Student’s t-test. *; p ≤ 0.05
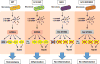
Figure 7
A schematic illustration of STING signaling responsible for the generation of pro-inflammatory cytokines to prevents colitis-associated with IL-10 deficiency.
Similar articles
- Role of toll-like receptors in spontaneous commensal-dependent colitis.
Rakoff-Nahoum S, Hao L, Medzhitov R. Rakoff-Nahoum S, et al. Immunity. 2006 Aug;25(2):319-29. doi: 10.1016/j.immuni.2006.06.010. Epub 2006 Aug 3. Immunity. 2006. PMID: 16879997 - MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice.
Hoshi N, Schenten D, Nish SA, Walther Z, Gagliani N, Flavell RA, Reizis B, Shen Z, Fox JG, Iwasaki A, Medzhitov R. Hoshi N, et al. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. Nat Commun. 2012. PMID: 23047678 Free PMC article. - Lactobacillus rhamnosus GG induces STING-dependent IL-10 in intestinal monocytes and alleviates inflammatory colitis in mice.
Si W, Zhao X, Li R, Li Y, Ma C, Zhao X, Bugno J, Qin Y, Zhang J, Liu H, Wang L. Si W, et al. J Clin Invest. 2025 Feb 3;135(3):e174910. doi: 10.1172/JCI174910. J Clin Invest. 2025. PMID: 39895628 Free PMC article. - cGAS-STING signaling pathway in intestinal homeostasis and diseases.
Yang Y, Wang L, Peugnet-González I, Parada-Venegas D, Dijkstra G, Faber KN. Yang Y, et al. Front Immunol. 2023 Sep 14;14:1239142. doi: 10.3389/fimmu.2023.1239142. eCollection 2023. Front Immunol. 2023. PMID: 37781354 Free PMC article. Review. - Inflammatory Bowel Disease (IBD)-Associated Colorectal Cancer (CRC): Is cGAS-STING Pathway Targeting the Key to Chemoprevention?
Papadakos SP, Georgiadou C, Argyrou A, Michailidou E, Thanos C, Vogli S, Siakavellas SI, Manolakopoulos S, Theocharis S. Papadakos SP, et al. Int J Mol Sci. 2025 May 22;26(11):4979. doi: 10.3390/ijms26114979. Int J Mol Sci. 2025. PMID: 40507791 Free PMC article. Review.
Cited by
- Discovery and characterization of a novel cGAS covalent inhibitor for the treatment of inflammatory bowel disease.
Song J, Yang RR, Chang J, Liu YD, Lu CH, Chen LF, Guo H, Zhang YH, Fan ZS, Zhou JY, Zhou GZ, Zhang KK, Luo XM, Chen KX, Jiang HL, Zhang SL, Zheng MY. Song J, et al. Acta Pharmacol Sin. 2023 Apr;44(4):791-800. doi: 10.1038/s41401-022-01002-5. Epub 2022 Oct 13. Acta Pharmacol Sin. 2023. PMID: 36229599 Free PMC article. - STING controls intestinal homeostasis through promoting antimicrobial peptide expression in epithelial cells.
Yu Y, Yang W, Bilotta AJ, Yu Y, Zhao X, Zhou Z, Yao S, Xu J, Zhou J, Dann SM, Li Y, Cong Y. Yu Y, et al. FASEB J. 2020 Nov;34(11):15417-15430. doi: 10.1096/fj.202001524R. Epub 2020 Sep 24. FASEB J. 2020. PMID: 32969062 Free PMC article. - Targeted immune activation in pediatric solid tumors: opportunities to complement local control approaches.
Vonderhaar EP, Dwinell MB, Craig BT. Vonderhaar EP, et al. Front Immunol. 2023 Jun 22;14:1202169. doi: 10.3389/fimmu.2023.1202169. eCollection 2023. Front Immunol. 2023. PMID: 37426669 Free PMC article. Review. - Intrinsic STING Switches off Pathogenetic Programs of Th1 Cells to Inhibit Colitis.
Yang W, Yu T, Zhou G, Yao S, Wakamiya M, Hu H, Paessler S, Sun J, Cong Y. Yang W, et al. Cell Mol Gastroenterol Hepatol. 2023;15(5):1161-1179. doi: 10.1016/j.jcmgh.2023.01.010. Epub 2023 Feb 2. Cell Mol Gastroenterol Hepatol. 2023. PMID: 36736893 Free PMC article. - The cGAS-STING pathway in diabetic retinopathy and age-related macular degeneration.
Hu B, Ma JX, Duerfeldt AS. Hu B, et al. Future Med Chem. 2023 Apr;15(8):717-729. doi: 10.4155/fmc-2022-0301. Epub 2023 May 11. Future Med Chem. 2023. PMID: 37166075 Free PMC article. Review.
References
- Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials