Involvements of p38 MAPK and oxidative stress in the ozone-induced enhancement of AHR and pulmonary inflammation in an allergic asthma model - PubMed (original) (raw)
Involvements of p38 MAPK and oxidative stress in the ozone-induced enhancement of AHR and pulmonary inflammation in an allergic asthma model
Aihua Bao et al. Respir Res. 2017.
Abstract
Background: Exposure to ambient ozone (O3) increases the susceptivity to allergens and triggers exacerbations in patients with asthma. However, the detailed mechanisms of action for O3 to trigger asthma exacerbations are still unclear.
Methods: An ovalbumin (OVA)-established asthmatic mouse model was selected to expose to filtered air (OVA-model) or 1.0 ppm O3 (OVA-O3 model) during the process of OVA challenge. Next, the possible involvements of p38 MAPK and oxidative stress in the ozone actions on the asthma exacerbations were investigated on the mice of OVA-O3 model by treating them with SB239063 (a p38 MAPK inhibitor), and/or the α-tocopherol (antioxidant). Biological measurements were conducted including airway hyperresponsiveness (AHR), airway resistance (Raw), lung compliance (CL), inflammation in the airway lumen and lung parenchyma, the phosphorylation of p38 MAPK and heat shock protein (HSP) 27 in the tracheal tissues, and the malondialdehyde (MDA) content and the glutathione peroxidase (GSH-Px) activity in lung tissues.
Results: In OVA-allergic mice, O3 exposure deteriorated airway hyperresponsiveness (AHR), airway resistance (Raw), lung compliance (CL) and pulmonary inflammation, accompanied by the increased oxidative stress in lung tissues and promoted p38 MAPK and HSP27 phosphorylation in tracheal tissues. Administration of SB239063 (a p38 MAPK inhibitor) on OVA-O3 model exclusively mitigated the Raw, the CL, and the BAL IL-13 content, while α-tocopherol (antioxidant) differentially reduced the BAL number of eosinophils and macrophages, the content of BAL hyaluronan, the peribronchial inflammation, as well as the mRNA expression of TNF-α and IL-5 in the lung tissues of OVA-O3 model. Administration of these two chemical inhibitors similarly inhibited the AHR, the BAL IFN-γ and IL-6 production, the perivascular lung inflammation and the lung IL-17 mRNA expression of OVA-O3 model. Interestingly, the combined treatment of both compounds together synergistically inhibited neutrophil counts in the BALF and CXCL-1 gene expression in the lung.
Conclusions: O3 exposure during the OVA challenge process promoted exacerbation in asthma. Both p38 MAPK and oxidative stress were found to play a critical role in this process and simultaneous inhibition of these two pathways significantly reduced the O3-elicited detrimental effects on the asthma exacerbation.
Keywords: Asthma exacerbation; Oxidative stress; Ozone; p38 MAPK; α-tocopherol.
Conflict of interest statement
Ethics approval and consent to participate
The protocol of this study was approved by the Shanghai General Hospital Institutional Review Board (Permit Number: 2010KY047).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
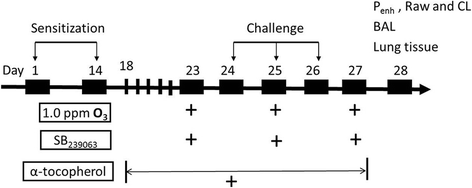
Fig. 1
Schematic diagram of the experimental protocol. Mice were sensitized at day 1 and 14, and challenged with OVA or saline at day 24, 25 and 26. Equal number of mice were exposed to 1.0 ppm O3 or filtered air for 3 h on day 23, 25 and 27. In a separate experiment, same number of O3-exposed asthmatic mice were injected through tail vein with SB239063 (4 mg/kg, diluted with DMSO) prior to each O3 exposure, or orally fed by gavage with α-tocopherol (15 IU/kg, diluted in 50% ethanol) for 10 consecutive days (from day 18 to day 27), or received them both. On day 28, measurements were performed including enhanced pause (Penh), airway resistance (Raw), lung compliance (CL), cell counts and cytokines in the BALF, mRNA expression of cytokines in the lung tissues, histological evaluation, p38 MAPK-HSP27 signaling by immunoblotting, and oxidative stress
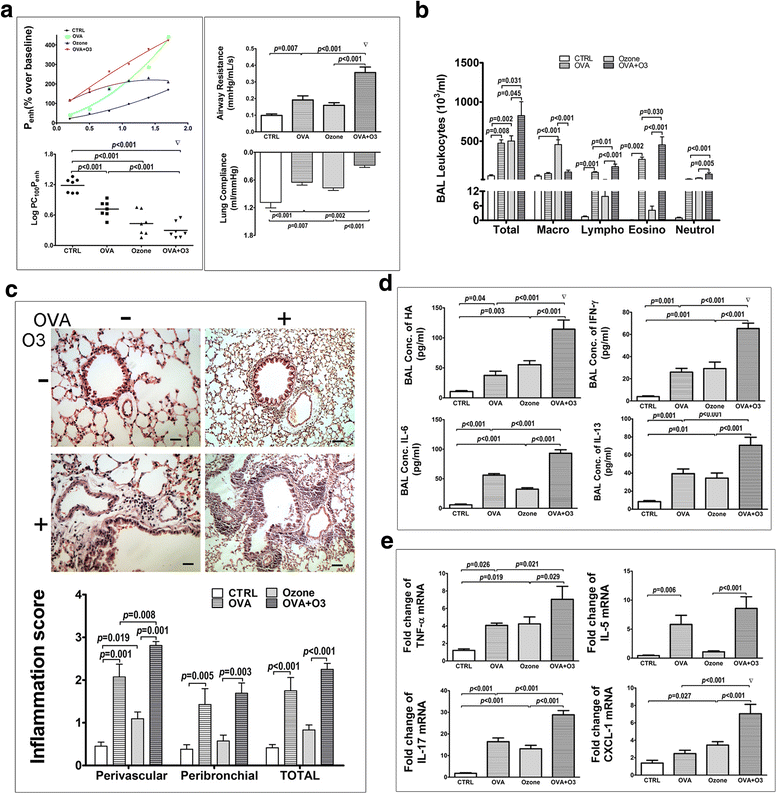
Fig. 2
Effects of O3 exposure on the allergic asthma model. a The lung function was assessed by the AHR in the left panels with the dose-response curves of Penh (top) and the log concentrations of methacholine required to increase Penh by 100% from baseline (LogPC100Penh) (bottom), the airway resistance (top right) and the lung compliance (bottom right). b The total and differentiated cell counts in the BALF. c The representative images of H&E stained lung sections (top) and histological inflammation scores (bottom) were shown. d cytokines (IL-6, IL-13 and IFN-γ) and HA concentration in the BALF. e mRNAs expression of TNF-α, IL-5, IL-17 and CXCL-1 in the lungs. Data are shown as mean ± SEM, n = 7 in each group. Synergistic effect of O3 and OVA, ▽:p < 0.05
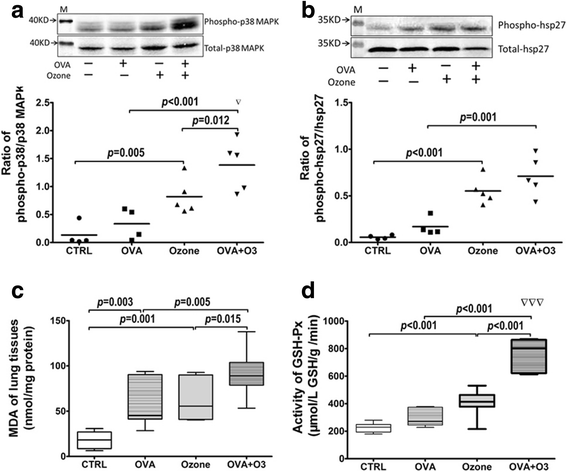
Fig. 3
The mechanism(s) of action of O3 on OVA-sensitized mice. O3 exposure affected the phosphorylation of p38 MAPK (a) and its downstream HSP27 (b) in tracheal tissues; n = 4 in control and OVA groups, n = 5 in ozone and OVA + O3 groups. The oxidative stress was evaluated by the content of malondialdehyde (MDA) (c) and the activity of glutathione peroxidase (GSH-Px) (d) in lung homogenates (n = 7 in each group). Data are shown as mean ± SEM. Synergistic effect of O3 and OVA, ▽:p < 0.05; ▽▽▽: p < 0.001
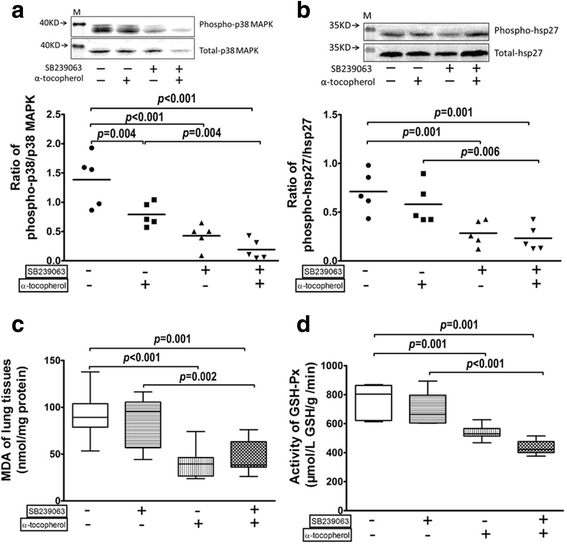
Fig. 4
Effect of SB239063 and α-tocopherol treatment on OVA-O3 mice model. Equal numbers of OVA-O3 mice were received tail vein injection of SB239063 (4 mg/kg) (DMSO as control), or oral feeding of α-tocopherol (15 IU/kg) (50% ethanol as control), or both. The phosphorylation of p38 MAPK (a) and HSP27 (b) in tracheal tissues were measured by immunoblotting; the MDA content (c) and the GSH-Px activity (d) in lung homogenates were analyzed to assess the oxidative stress. Data are shown as mean ± SEM, n = 5 in each group
Fig. 5
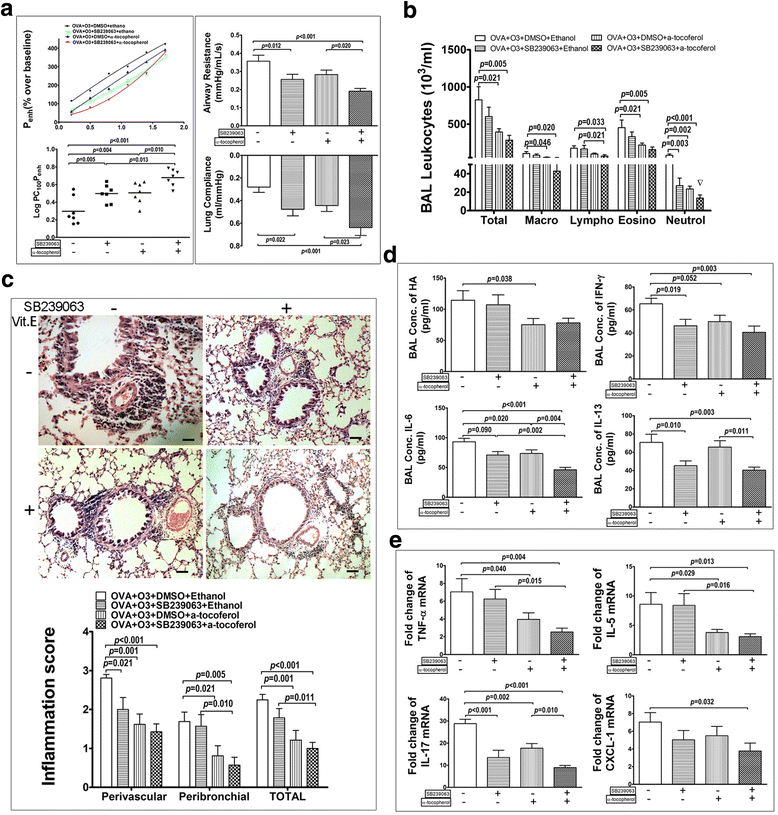
Role of p38 MAPK and oxidative stress in the ozonic effects on OVA challenged mice. OVA-O3 mice were received tail vein injection of SB239063 (4 mg/kg) (DMSO as control), or oral feeding of α-tocopherol (15 IU/kg) (50% ethanol as control), or both. a The lung function was assessed by the AHR in the left panels with the dose-response curves of Penh (top) and the log concentrations of methacholine required to increase Penh by 100% from baseline (LogPC100Penh) (bottom) showed, the airway resistance (top right) and the lung compliance (bottom right). b The total and differential cell counts in the BALF. c The representative images of H&E stained lung sections (top) and histological inflammation scores (bottom). d cytokines (IL-6, IL-13 and IFN-γ) and HA concentration in the BALF. e mRNAs expression of TNF-α, IL-5, IL-17 and CXCL-1 in the lungs. Data are shown as mean ± SEM; n = 7 in each group. Synergistic effect of SB239063& α-tocopherol, ▽:p < 0.05
Similar articles
- Activation of p38 mitogen-activated protein kinase in ovalbumin and ozone-induced mouse model of asthma.
Liang L, Li F, Bao A, Zhang M, Chung KF, Zhou X. Liang L, et al. Respirology. 2013 Nov;18 Suppl 3:20-9. doi: 10.1111/resp.12189. Respirology. 2013. PMID: 24188200 - Impact of ozone exposure on the response to glucocorticoid in a mouse model of asthma: involvements of p38 MAPK and MKP-1.
Bao A, Li F, Zhang M, Chen Y, Zhang P, Zhou X. Bao A, et al. Respir Res. 2014 Oct 8;15(1):126. doi: 10.1186/s12931-014-0126-x. Respir Res. 2014. PMID: 25287866 Free PMC article. - Effects of ozone repeated short exposures on the airway/lung inflammation, airway hyperresponsiveness and mucus production in a mouse model of ovalbumin-induced asthma.
Bao W, Zhang Y, Zhang M, Bao A, Fei X, Zhang X, Zhou X. Bao W, et al. Biomed Pharmacother. 2018 May;101:293-303. doi: 10.1016/j.biopha.2018.02.079. Epub 2018 Feb 27. Biomed Pharmacother. 2018. PMID: 29499403 - Ozone-induced airway hyperresponsiveness: roles of ROCK isoforms.
Lambert JA, Song W. Lambert JA, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Dec 15;309(12):L1394-7. doi: 10.1152/ajplung.00353.2015. Epub 2015 Oct 30. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26519207 Review. - Mechanistic Basis for Obesity-related Increases in Ozone-induced Airway Hyperresponsiveness in Mice.
Shore SA. Shore SA. Ann Am Thorac Soc. 2017 Nov;14(Supplement_5):S357-S362. doi: 10.1513/AnnalsATS.201702-140AW. Ann Am Thorac Soc. 2017. PMID: 29161088 Free PMC article. Review.
Cited by
- Network pharmacology and experimental verification unraveled the mechanism of Bailing Capsule against asthma.
Lin S, Chen M, Lin S, Huang X, Chen W, Wu S. Lin S, et al. Medicine (Baltimore). 2024 Nov 1;103(44):e40391. doi: 10.1097/MD.0000000000040391. Medicine (Baltimore). 2024. PMID: 39495985 Free PMC article. - Natural and socio-environmental factors in the transmission of COVID-19: a comprehensive analysis of epidemiology and mechanisms.
Gong Z, Song T, Hu M, Che Q, Guo J, Zhang H, Li H, Wang Y, Liu B, Shi N. Gong Z, et al. BMC Public Health. 2024 Aug 13;24(1):2196. doi: 10.1186/s12889-024-19749-3. BMC Public Health. 2024. PMID: 39138466 Free PMC article. Review. - Green tea extract suppresses airway inflammation via oxidative stress-driven MAPKs/MMP-9 signaling in asthmatic mice and human airway epithelial cells.
Kim JW, Kim JH, Jeong JS, Kim CY, Chung EH, Kim SH, Hong EJ, Kwon HJ, Ko JW, Kim TW. Kim JW, et al. Front Immunol. 2024 Apr 30;15:1362404. doi: 10.3389/fimmu.2024.1362404. eCollection 2024. Front Immunol. 2024. PMID: 38745671 Free PMC article. - Involvement of ERK and Oxidative Stress in Airway Exposure to Cadmium Chloride Aggravates Airway Inflammation in Ovalbumin-Induced Asthmatic Mice.
Wu C, Hu X, Jiang Y, Tang J, Ge H, Deng S, Li X, Feng J. Wu C, et al. Toxics. 2024 Mar 23;12(4):235. doi: 10.3390/toxics12040235. Toxics. 2024. PMID: 38668459 Free PMC article. - "Air That Once Was Breath" Part 1: Wildfire-Smoke-Induced Mechanisms of Airway Inflammation - "Climate Change, Allergy and Immunology" Special IAAI Article Collection: Collegium Internationale Allergologicum Update 2023.
Bowman WS, Schmidt RJ, Sanghar GK, Thompson Iii GR, Ji H, Zeki AA, Haczku A. Bowman WS, et al. Int Arch Allergy Immunol. 2024;185(6):600-616. doi: 10.1159/000536578. Epub 2024 Mar 7. Int Arch Allergy Immunol. 2024. PMID: 38452750 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 201440296/Shanghai Municipal Commission of Health and Family Planning grant/International
- 13XJI0063/Shanghai Jiao Tong University School of Medicine grant/International
- 81570018/National Nature Science Foundation of China/International
- 81470218/National Nature Science Foundation of China/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous