Cost-effectiveness of the Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults - PubMed (original) (raw)
Cost-effectiveness of the Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults
Phuc Le et al. JAMA Intern Med. 2018.
Abstract
Importance: The live attenuated herpes zoster vaccine (ZVL) is recommended for immunocompetent adults 60 years or older, but the efficacy wanes with age and over time. A new adjuvanted herpes zoster subunit vaccine (HZ/su) has higher efficacy but might be more expensive. The choice of vaccines depends on their relative values.
Objective: To assess the cost-effectiveness of HZ/su.
Design, setting, and participants: Markov decision model with transition probabilities based on the US medical literature. Participants were immunocompetent adults 60 years or older. Data were derived from participant groups ranging in number from less than 100 to more than 30 000 depending on the variable assessed. The study dates were July 1 to 31, 2017.
Exposures: No vaccination, ZVL (single dose), and HZ/su (2-dose series) vaccine administered at different ages.
Main outcomes and measures: Total costs and quality-adjusted life-years (QALYs) were estimated.
Results: Based on randomized clinical trial data, at a price of 280perseries(280 per series (280perseries(140 per dose), HZ/su was more effective and less expensive than ZVL at all ages. The incremental cost-effectiveness ratios compared with no vaccination ranged from 20038to20 038 to 20038to30 084 per QALY, depending on vaccination age. The finding was insensitive to variations in most model inputs other than the vaccine price and certain combinations of low adherence rate with a second dose and low efficacy of a single dose of HZ/su. At the current ZVL price ($213 per dose), HZ/su had lower overall costs than ZVL up to a price of 350per2−doseseries.Inprobabilisticsensitivityanalysis,HZ/suhad73350 per 2-dose series. In probabilistic sensitivity analysis, HZ/su had 73% probability of being cost-effective for 60-year-olds at 350per2−doseseries.Inprobabilisticsensitivityanalysis,HZ/suhad7350 000 per QALY.
Conclusions and relevance: Under conservative assumptions, at a price of 280perseries(280 per series (280perseries(140 per dose), HZ/su would cost less than ZVL and has a high probability of offering good value.
Conflict of interest statement
Conflict of Interest Disclosures: None reported.
Figures
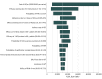
Figure 1.. Tornado Diagram of the Incremental Cost-effectiveness Ratio (ICER) of the Adjuvanted Herpes Zoster Subunit Vaccine (HZ/su) at Vaccination Age of 60 Years
Ranges are in parentheses, with the left values leading to the leftmost ICERs. BOI indicates burden of illness; HZ, herpes zoster; PHN, postherpetic neuralgia; QALY, quality-adjusted life-year; and ZVL, live attenuated herpes zoster vaccine. ICER values are given in 2016 US dollars. aRanges for these age-specific parameters are listed in Table 1, with the highest values leading to the leftmost ICERs. bIncidences in both men and women were varied at the same time with ranges listed in Table 1.
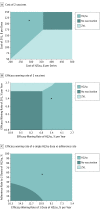
Figure 2.. Two-Way Sensitivity Analysis at Vaccination Age of 60 Years With a Willingness-to-Pay Threshold of $50 000 per Quality-Adjusted Life-year
A-C, Letter x denotes base case. HZ/su indicates adjuvanted herpes zoster subunit vaccine; ZVL, live attenuated herpes zoster vaccine. Costs are given in 2016 US dollars.
Figure 3.. Probability of Each Strategy Being Cost-effective at Different Willingness-to-Pay Thresholds
A-C, Probabilistic sensitivity analysis. HZ/su indicates adjuvanted herpes zoster subunit vaccine; QALY, quality-adjusted life-year; and ZVL, live attenuated herpes zoster vaccine. Costs are given in 2016 US dollars.
Comment in
- Evolution of Herpes Zoster Vaccines and Their Economic Value.
Najafzadeh M. Najafzadeh M. JAMA Intern Med. 2018 Feb 1;178(2):258-259. doi: 10.1001/jamainternmed.2017.7442. JAMA Intern Med. 2018. PMID: 29297047 No abstract available. - Cost-effectiveness of the Adjuvanted Herpes Zoster Subunit Vaccine.
Good CB, Hernandez I. Good CB, et al. JAMA Intern Med. 2018 Jun 1;178(6):873. doi: 10.1001/jamainternmed.2018.2029. JAMA Intern Med. 2018. PMID: 29868745 No abstract available.
Similar articles
- Cost-effectiveness of vaccination of immunocompetent older adults against herpes zoster in the Netherlands: a comparison between the adjuvanted subunit and live-attenuated vaccines.
de Boer PT, van Lier A, de Melker H, van Wijck AJM, Wilschut JC, van Hoek AJ, Postma MJ. de Boer PT, et al. BMC Med. 2018 Dec 6;16(1):228. doi: 10.1186/s12916-018-1213-5. BMC Med. 2018. PMID: 30518427 Free PMC article. - Assessment of the potential public health impact of Herpes Zoster vaccination in Germany.
Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, von Krempelhuber A, Anastassopoulou A. Curran D, et al. Hum Vaccin Immunother. 2017 Oct 3;13(10):2213-2221. doi: 10.1080/21645515.2017.1345399. Epub 2017 Jul 14. Hum Vaccin Immunother. 2017. PMID: 28708959 Free PMC article. - A systematic review of the cost effectiveness of herpes zoster vaccination.
Szucs TD, Pfeil AM. Szucs TD, et al. Pharmacoeconomics. 2013 Feb;31(2):125-36. doi: 10.1007/s40273-012-0020-7. Pharmacoeconomics. 2013. PMID: 23335045 Review. - Herpes zoster subunit vaccine for the prevention of herpes zoster.
Symoniak MR, Farrokh P, Gandhi MA, Slish JC. Symoniak MR, et al. Am J Health Syst Pharm. 2018 Jun 15;75(12):861-869. doi: 10.2146/ajhp170399. Am J Health Syst Pharm. 2018. PMID: 29880523 Review.
Cited by
- Cost-effectiveness of varicella and herpes zoster vaccination in Sweden: An economic evaluation using a dynamic transmission model.
Wolff E, Widgren K, Scalia Tomba G, Roth A, Lep T, Andersson S. Wolff E, et al. PLoS One. 2021 May 13;16(5):e0251644. doi: 10.1371/journal.pone.0251644. eCollection 2021. PLoS One. 2021. PMID: 33984060 Free PMC article. - The potential public health impact of Herpes Zoster vaccination in the 65 years of age cohort in Italy.
Volpi A, Boccalini S, Dari S, Clarke C, Curran D, Loiacono I, Pitrelli A, Puggina A, Tosatto R, Van Oorschot D, Franco E. Volpi A, et al. Hum Vaccin Immunother. 2020;16(2):327-334. doi: 10.1080/21645515.2019.1657753. Epub 2019 Sep 24. Hum Vaccin Immunother. 2020. PMID: 31442095 Free PMC article. - Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany.
Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Curran D, et al. Hum Vaccin Immunother. 2021 Dec 2;17(12):5296-5303. doi: 10.1080/21645515.2021.2002085. Epub 2021 Dec 14. Hum Vaccin Immunother. 2021. PMID: 34905463 Free PMC article. - A Cost-effectiveness Analysis of an Adjuvanted Subunit Vaccine for the Prevention of Herpes Zoster and Post-herpetic Neuralgia.
Carpenter CF, Aljassem A, Stassinopoulos J, Pisacreta G, Hutton D. Carpenter CF, et al. Open Forum Infect Dis. 2019 May 8;6(7):ofz219. doi: 10.1093/ofid/ofz219. eCollection 2019 Jul. Open Forum Infect Dis. 2019. PMID: 31289726 Free PMC article. - Effectiveness and cost-effectiveness of vaccination against herpes zoster in Canada: a modelling study.
Drolet M, Zhou Z, Sauvageau C, DeWals P, Gilca V, Amini R, Bénard É, Brisson M. Drolet M, et al. CMAJ. 2019 Aug 26;191(34):E932-E939. doi: 10.1503/cmaj.190274. CMAJ. 2019. PMID: 31451524 Free PMC article.
References
- Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284. - PubMed
- Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087-2096. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical