Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer's Disease Pathogenesis - PubMed (original) (raw)
Review
Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer's Disease Pathogenesis
Duraisamy Kempuraj et al. Front Neurosci. 2017.
Abstract
Mast cells are localized throughout the body and mediate allergic, immune, and inflammatory reactions. They are heterogeneous, tissue-resident, long-lived, and granulated cells. Mast cells increase their numbers in specific site in the body by proliferation, increased recruitment, increased survival, and increased rate of maturation from its progenitors. Mast cells are implicated in brain injuries, neuropsychiatric disorders, stress, neuroinflammation, and neurodegeneration. Brain mast cells are the first responders before microglia in the brain injuries since mast cells can release prestored mediators. Mast cells also can detect amyloid plaque formation during Alzheimer's disease (AD) pathogenesis. Stress conditions activate mast cells to release prestored and newly synthesized inflammatory mediators and induce increased blood-brain barrier permeability, recruitment of immune and inflammatory cells into the brain and neuroinflammation. Stress induces the release of corticotropin-releasing hormone (CRH) from paraventricular nucleus of hypothalamus and mast cells. CRH activates glial cells and mast cells through CRH receptors and releases neuroinflammatory mediators. Stress also increases proinflammatory mediator release in the peripheral systems that can induce and augment neuroinflammation. Post-traumatic stress disorder (PTSD) is a traumatic-chronic stress related mental dysfunction. Currently there is no specific therapy to treat PTSD since its disease mechanisms are not yet clearly understood. Moreover, recent reports indicate that PTSD could induce and augment neuroinflammation and neurodegeneration in the pathogenesis of neurodegenerative diseases. Mast cells play a crucial role in the peripheral inflammation as well as in neuroinflammation due to brain injuries, stress, depression, and PTSD. Therefore, mast cells activation in brain injury, stress, and PTSD may accelerate the pathogenesis of neuroinflammatory and neurodegenerative diseases including AD. This review focusses on how mast cells in brain injuries, stress, and PTSD may promote the pathogenesis of AD. We suggest that inhibition of mast cells activation and brain cells associated inflammatory pathways in the brain injuries, stress, and PTSD can be explored as a new therapeutic target to delay or prevent the pathogenesis and severity of AD.
Keywords: Alzheimer's disease; PTSD; mast cells; neurodegeneration; neuroinflammation; stress.
Figures
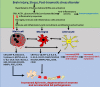
Figure 1
Schematic diagram showing mast cells, brain injury, stress, and PTSD in neurodegeneration in AD pathogenesis. Mast cells-derived inflammatory mediators and direct contact of mast cells with glial cells and neurons, activates brain cells to release additional neuroinflammatory mediators that induce neurodegeneration. Stress conditions activate mast cells through CRH and other neuropeptides to release several neuroinflammatory mediators including cytokines, chemokines, and other neurotoxic mediators such as tryptase, histamine, IL-1β, TNF-α, IL-6, CCL2, IL-8, ROS, CRH, and MMPs. These inflammatory mediators in turn activate glial cells to release additional inflammatory mediators and induce neurodegeneration and neuronal death. Stress also increases BBB permeability and increases the recruitment of immune and inflammatory cells such as mast cell progenitors, mast cells, and T cells in to the brain. In addition, mast cell mediators released from the activated mast cells increase the vascular permeability, increase recruitment of immune, and inflammatory cells to the site of injury and induce neuroinflammatory reactions. These newly recruited mast cell progenitors, mast cells, and T-cells proliferate, mature, and further activate glial cells and neurons by releasing inflammatory and cytotoxic mediators leading to chronic state of neuroinflammation and neurodegeneration. PTSD also induces neuroinflammation and neurodegeneration through immune and inflammatory cells such as mast cells, T-cells, and glial cells in the brain. Mast cells, brain injury, stress conditions, and PTSD comorbidity could induce chronic neuroinflammation and neurodegeneration in neurodegenerative diseases including AD.
Similar articles
- Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration.
Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A. Kempuraj D, et al. Front Cell Neurosci. 2017 Jul 24;11:216. doi: 10.3389/fncel.2017.00216. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28790893 Free PMC article. Review. - Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer's Disease.
Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A. Kempuraj D, et al. Front Cell Neurosci. 2019 Feb 19;13:54. doi: 10.3389/fncel.2019.00054. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30837843 Free PMC article. - Psychological Stress-Induced Immune Response and Risk of Alzheimer's Disease in Veterans from Operation Enduring Freedom and Operation Iraqi Freedom.
Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Raikwar SP, Zaheer SA, Iyer SS, Burton C, James D, Zaheer A. Kempuraj D, et al. Clin Ther. 2020 Jun;42(6):974-982. doi: 10.1016/j.clinthera.2020.02.018. Epub 2020 Mar 14. Clin Ther. 2020. PMID: 32184013 Free PMC article. Review. - Brain Injury-Mediated Neuroinflammatory Response and Alzheimer's Disease.
Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, Dubova I, Mentor S, Premkumar K, Saeed D, Zahoor H, Raikwar SP, Zaheer S, Iyer SS, Zaheer A. Kempuraj D, et al. Neuroscientist. 2020 Apr;26(2):134-155. doi: 10.1177/1073858419848293. Epub 2019 May 16. Neuroscientist. 2020. PMID: 31092147 Free PMC article. Review. - Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma.
Kempuraj D, Thangavel R, Kempuraj DD, Ahmed ME, Selvakumar GP, Raikwar SP, Zaheer SA, Iyer SS, Govindarajan R, Chandrasekaran PN, Zaheer A. Kempuraj D, et al. Biofactors. 2021 Mar;47(2):190-197. doi: 10.1002/biof.1687. Epub 2020 Oct 24. Biofactors. 2021. PMID: 33098588 Review.
Cited by
- c-KIT inhibitors reduce pathology and improve behavior in the Tg(SwDI) model of Alzheimer's disease.
Stevenson M, Hebron ML, Liu X, Balaraman K, Wolf C, Moussa C. Stevenson M, et al. Life Sci Alliance. 2024 Jul 15;7(10):e202402625. doi: 10.26508/lsa.202402625. Print 2024 Oct. Life Sci Alliance. 2024. PMID: 39009412 Free PMC article. - P2X Receptor-Dependent Modulation of Mast Cell and Glial Cell Activities in Neuroinflammation.
Salcman B, Affleck K, Bulfone-Paus S. Salcman B, et al. Cells. 2021 Sep 2;10(9):2282. doi: 10.3390/cells10092282. Cells. 2021. PMID: 34571930 Free PMC article. Review. - Potential Role of Moesin in Regulating Mast Cell Secretion.
Theoharides TC, Kempuraj D. Theoharides TC, et al. Int J Mol Sci. 2023 Jul 28;24(15):12081. doi: 10.3390/ijms241512081. Int J Mol Sci. 2023. PMID: 37569454 Free PMC article. Review. - Recent Research Trends in Neuroinflammatory and Neurodegenerative Disorders.
Cohen J, Mathew A, Dourvetakis KD, Sanchez-Guerrero E, Pangeni RP, Gurusamy N, Aenlle KK, Ravindran G, Twahir A, Isler D, Sosa-Garcia SR, Llizo A, Bested AC, Theoharides TC, Klimas NG, Kempuraj D. Cohen J, et al. Cells. 2024 Mar 14;13(6):511. doi: 10.3390/cells13060511. Cells. 2024. PMID: 38534355 Free PMC article. Review. - Neurodivergence, intersectionality, and eating disorders: a lived experience-led narrative review.
Cobbaert L, Millichamp AR, Elwyn R, Silverstein S, Schweizer K, Thomas E, Miskovic-Wheatley J. Cobbaert L, et al. J Eat Disord. 2024 Nov 20;12(1):187. doi: 10.1186/s40337-024-01126-5. J Eat Disord. 2024. PMID: 39568093 Free PMC article. Review.
References
- Ahmed M. E., Iyer S., Thangavel R., Kempuraj D., Selvakumar G. P., Raikwar S. P., et al. . (2017). Co-Localization of glia maturation factor with NLRP3 inflammasome and autophagosome markers in human Alzheimer's disease brain. J. Alzheimers Dis. 60, 1143–1160. 10.3233/JAD-170634 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources