Drug-Driven Phenotypic Convergence Supports Rational Treatment Strategies of Chronic Infections - PubMed (original) (raw)
Drug-Driven Phenotypic Convergence Supports Rational Treatment Strategies of Chronic Infections
Lejla Imamovic et al. Cell. 2018.
Abstract
Chronic Pseudomonas aeruginosa infections evade antibiotic therapy and are associated with mortality in cystic fibrosis (CF) patients. We find that in vitro resistance evolution of P. aeruginosa toward clinically relevant antibiotics leads to phenotypic convergence toward distinct states. These states are associated with collateral sensitivity toward several antibiotic classes and encoded by mutations in antibiotic resistance genes, including transcriptional regulator nfxB. Longitudinal analysis of isolates from CF patients reveals similar and defined phenotypic states, which are associated with extinction of specific sub-lineages in patients. In-depth investigation of chronic P. aeruginosa populations in a CF patient during antibiotic therapy revealed dramatic genotypic and phenotypic convergence. Notably, fluoroquinolone-resistant subpopulations harboring nfxB mutations were eradicated by antibiotic therapy as predicted by our in vitro data. This study supports the hypothesis that antibiotic treatment of chronic infections can be optimized by targeting phenotypic states associated with specific mutations to improve treatment success in chronic infections.
Keywords: Pseudomonas aeruginosa; antibiotic treatment; chronic infections; collateral sensitivity; cystic fibrosis; drug resistance; nfxB; phenotypic convergence.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure S1
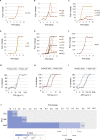
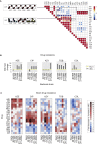
Selection for Resistance during Laboratory Evolution Experiments and Collateral Changes in Susceptibility Profiles, Related to Figure 1 (A–F) Increase in antibiotic resistance during adaptive evolution experiment in SCFM. Fold increase depicts the well from each day transfer was made in antibiotic gradient plate. Fold increase is calculated relative to the day 1. (G–J) Resistance that alters the collateral sensitivity profiles of parallel evolved PAO1 strains. Three outcomes of resistance development were observed: (G) no change in susceptibility profiles relative to the WTE (black line), (H) collateral resistance or decrease in drug susceptibility relative to the WTE (red line) and (I) collateral sensitivity or increase in drug susceptibility relative to the WTE (blue line). For each strain, five replicates were performed to determine the drug susceptibility (±SD, error bars). (J) Significance test of the fold of difference in resistance between the WTE and ciprofloxacin resistant strain. Using the mean and standard deviation of the five replicates, growth inhibition of ciprofloxacin resistant strains toward three different antibiotics was calculated. _P_-value was determined using t test test with alternative hypothesis “greater” for the fold increase between one and 10 (by factor 0.5).
Figure 1
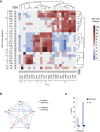
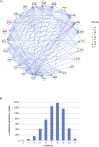
Consequence of Drug Resistance Evolution: Collateral Sensitivity and Collateral Resistance (A) Heatmap represents quantification the collateral sensitivity profiles of the 24 evolved antibiotic-resistant PAO1 strains. Color coding represents the fold increase (red) or decrease (blue) in MIC value relative to the PAO1 strain evolved in SCFM media without antibiotics (WTE). For each strain, five replicates dose-response curves were performed to determine the drug susceptibility (Table S1). The order of the drugs and resistant strains was determined by hierarchical clustering using the similarity of normalized MIC values as the distance measure. (B) Sub-network of collateral interactions among drugs commonly administered in treatments of CF patients. For collateral susceptibility networks, the directed path of each arrow represents the collateral sensitivity (blue) or collateral resistance (red) of an affected variable (drug-resistant strain) on the causal variable (drug). Collateral sensitivity cycling for two drugs would consist of alternating application of two drugs with collateral sensitivity (e.g., colistin and aztreonam) (full network for collateral sensitivity and resistance interaction depicted in Figures S2A and S2B). (C) Number of possible collateral sensitivity cycles with anti-pseudomonal drugs (EUCAST) versus all drugs used in the study (Table S2). See also Figures S1 and S2.
Figure S2
Complex Networks of Interactions Based on the Collateral Susceptibility Profiles, Related to Figure 1 (A) Collateral sensitivity network. For collateral susceptibility networks, the directed path of each arrow represents the collateral sensitivity (blue) or collateral resistance (red) of an affected variable (drug-resistant strain) on the causal variable (drug). Antibiotic abbreviations are listed in Table 1. (B) Number of collateral sensitivity cycles simulated for all drugs employed in the study. The cycles are based on PAO1 susceptibility profiles (Figure 1A).
Figure S3
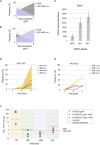
A Relationship between the Altered Susceptibilities of the Experimentally Evolved Resistant PAO1 and Collateral Sensitivity Profiles for DK2 Clinical Isolates, Related to Figure 2 (A) A Spearman correlation matrix for all pairwise susceptibility profiles of PAO1 drug-resistant strains. MIC values were normalized to WTE strain and log2 transformed. Upper right color panel is an indicator of the Spearman correlation coefficient (ρ). Circle size represents the strength of Spearman correlation’s coefficient (ρ.) Only statistically significant correlations are shown (p > 0.05, two-tailed test) (Table S3). Scatterplots represent a positive correlation between ciprofloxacin (CIP) and other strains resistant to four different chemical classes. The data displayed on the second scatterplot depict positive correlations (with aztreonam and two β-lactam resistant strains) and negative correlations (with aminoglycoside and polymyxin resistant strains). In addition, plots in yellow show strains for which no significant correlation was observed (p > 0.05, two-tailed test). (B) Initial susceptibility levels for five DK2 isolates selected for the adaptive evolution experiment. Data are presented as the MIC fold change relative to the DK2 strain not exposed to antibiotics. (C) Collateral sensitivity and resistance during adaptive evolution for DK2 drug resistant strains. The MIC values were normalized to the baseline susceptibilities of each WT for evolved DK2 isolates (WTE) (Table S1).
Figure 2
Drug Resistance Evolution Converges to Structural Phenotypic States (A) Principal component analysis of the susceptibility profiles of evolved antibiotic resistance PAO1 strains reveals clustering of resistance phenotypes to specific phenotypic states. Principal component axes obtained from the PAO1 WTE normalized susceptibility data for the resistant PAO1 strains. Color coding depicts the drug classes listed in Table 1. (B) Correlation analysis for PAO and DK2 susceptibility profiles. Exposure of different P. aeruginosa strains to a particular drug tends to increase the correlation between evolved antibiotic-resistant DK2 strains and PAO1 strains. Spearman’s correlation coefficients (ρ) summarize the pairwise correlative relationship between the altered susceptibility of drug resistant strains. Circle size represents the strength of ρ (Table S4). (C and D) Shifts in phenotypic states toward specific group collateral states drug-resistant DK2 strains toward their corresponding resistant PAO1 strain. Space plot of two principal component axes obtained from the PAO1 WTE normalized and log2 transformed susceptibility data for DK2 WTE and DK2 ciprofloxacin-resistant strain (C) or aztreonam-resistant strain (D). See also Figure S3.
Figure S4
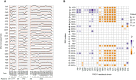
Mutational Events Leading to Drug Resistance for PAO1 and Changes in Proteome in Drug-Resistant Strains Sharing nfxB Mutation, Related to Figure 3 (A) Mutational events leading to drug resistance for PAO1 (Table S5). The order of the drugs and resistant strains was determined by hierarchical clustering using the shared mutation as a value for the distance measure. Antibiotic and class abbreviations are listed in Table 1. (B) Changes in proteome in drug ciprofloxacin and azithromycin resistant strains harboring nfxB mutation. Fold change for specific proteins was calculated based on the level of the proteins in WTE sample. t test were preformed to determine the ratio of proteins with significantly altered abundance (95% confidence). Only proteins with at least 1.5-fold increase (_P_-value < 0.05, t test) were reported. (C) MexCD-OprJ efflux is negatively regulated by NfxB repressor binding upstream of mexC gene. (D) Mutation in nfxB lead affects repressor binding leading to expression of MexCD-OprJ efflux system. Expression of MexCD-OprJ efflux system resulted in increased abundance of MexC protein leading to collateral sensitivity and resistance.
Figure 3
Genetic Basis for Collateral Resistance and Collateral Sensitivity (A) Competition experiment depicting the survival of WT over the resistant strain harboring the nfxB mutation when treated with collateral-sensitive antibiotic. (B) The selective survival of resistant strain and eradication of the WT was observed in competition experiment when treated with collateral-resistant antibiotic (C) MexC abundance in WTE, ciprofloxacin- and azithromycin-resistant strains. Data are presented as the means of three biological replicates and error bars represent SD. Significance levels indicate the p value of the t test (Table S6). (D) nfxB mutations in populations at after gyrA mutation during adaptive evolution to ciprofloxacin. (E) nfxB mutations at the end of the adaptive process for azithromycin exposed bacterial populations. (F) Changes in susceptibility profiles for 173-2005 relative to ancestral clinical isolated (30-1979) and upon further resistance development to CIP and AZY (Table S1). Dashed lines mark the EUCAST clinical resistance breakpoints (Table 1). See also Figure S4.
Figure 4
Oscillatory Dynamics in Susceptibility Profiles from P. aeruginosa Chronically Infected CF Patients (A) Susceptibility profiles for clinical isolates obtained by longitudinal sampling of DK2 clinical isolates from three CF patients chronically infected by P. aeruginosa. For each strain, MIC values were determined toward 22 drugs. Drug susceptibility was determined based on the average values of five replicates. MIC values were normalized to the baseline susceptibilities of the immediate common ancestor of the DK2 isolates (isolate 30-1979) (Table S1). (B) A heatmap of Spearman’s correlation coefficients (ρ) summarizes the pairwise correlative relationship between the altered susceptibilities of the experimentally evolved resistant PAO1 strains and DK2 isolates from CF patients. The upper-right color panel is an indicator of the Spearman correlation coefficient (ρ). Circle size represents the strength of ρ. Only statistically significant correlations are shown (p > 0.05, two-tailed test) (Table S6).
Figure 5
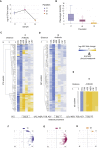
Shift in Susceptibility Profiles during Intensive Antibiotic Treatment In Vivo (A) Recovery of P. aeruginosa from sputum on selective media. Plating was done in five replicates for samples t1 and t2. For sample t3, 12 agar plates were used to recover P. aeruginosa. The line dots represent the average and error bars represent SD. (B) Decrease in overall phenotypic diversity during the course of antibiotic treatment. Change in phenotypic diversity was calculated based on Euclidian distance between the normalized values for susceptibility profiles. (C–E) Sensitivity profiles during antibiotic treatment of CF patient. Heatmap represents quantification of the drug response for single isolates obtained before (C), during (D), and at the end of treatment (E). Antibiotic and class abbreviations are listed in Table 1. Color coding represents the fold above (yellow) or below (blue) the isolate MIC value relative to the EUCAST clinical breakpoints (Table 1). Drug susceptibility was determined based on the average values of five replicates (Table S7). The order of isolates was determined by hierarchical clustering using the similarity of normalized MIC values as the distance measure. (F–H) Space plot of two principal component axes obtained from the 626 clinical isolates normalized susceptibility data for the EUCAST resistance breakpoints. Color coding depicts the sampling time points before (F), during (G), and at the end of (H) treatment. See also Figure S5.
Figure S5
Phenotypic Convergences for Heterogeneous P. aeruginosa Population during Antibiotic Treatment, Related to Figure 5 (A–F) Distribution plots for susceptibility profiles of clinical isolates toward six clinically relevant antibiotics. (G) PCA plot for antibiotic susceptibility of clinical isolates obtained before (t1), during (t2) and at the end (t3) of intensive antibiotic treatment of CF patient. (H) PCA plot for quinolone resistant strains before (t1-b) and at the end of intensive antibiotic treatment (t3) for CF patient. For all panels, normalized MIC values were used as data input. MIC or the inhibitory concentration was defined as the lowest concentration of the drug that inhibited 90% of the growth of the strain tested. For each strain, five replicates were performed to determine the drug susceptibility. All MIC data were normalized to EUCAST resistant breakpoint values and log2 transformed.
Figure 6
Population Switch during Antibiotic Treatment In Vivo (A) Population sequencing of clinical isolates. Individual and shared mutations were plotted and used to evaluate population divergence during treatment. Variable percentage of shared mutations was observed among different subpopulations before (t1-a and t1-b), during (t2), and at the end of intensive treatment (t3) (Table S9). By calculating that majority mutations found in quinolone-resistant population t3 subpopulation are shared with t1-b, population divergence was estimated. Different color coding represent mutation shared by different subpopulations (B) Genotype distance and susceptibility profiles among selected isolates. The order of isolates was determined by hierarchical clustering using the shared mutation as a value for the distance measure. Antibiotic and class abbreviations are listed in Table 1. Color coding represents the fold increase or decrease in MIC value relative to the EUCAST clinical breakpoints (Table 1). An average of five replicates were tested to determine the drug susceptibility (Table S1). (C) nfxB mutation frequency in quinolone-resistant subpopulations t1-a and t1-b. (D) Loss of resistant isolates harboring nfxB after exposure to β-lactam and aminoglycoside antibiotics during treatment of CF patient.
Similar articles
- Within-host whole genome analysis of an antibiotic resistant Pseudomonas aeruginosa strain sub-type in cystic fibrosis.
Sherrard LJ, Tai AS, Wee BA, Ramsay KA, Kidd TJ, Ben Zakour NL, Whiley DM, Beatson SA, Bell SC. Sherrard LJ, et al. PLoS One. 2017 Mar 8;12(3):e0172179. doi: 10.1371/journal.pone.0172179. eCollection 2017. PLoS One. 2017. PMID: 28273168 Free PMC article. - Evolved Aztreonam Resistance Is Multifactorial and Can Produce Hypervirulence in Pseudomonas aeruginosa.
Jorth P, McLean K, Ratjen A, Secor PR, Bautista GE, Ravishankar S, Rezayat A, Garudathri J, Harrison JJ, Harwood RA, Penewit K, Waalkes A, Singh PK, Salipante SJ. Jorth P, et al. mBio. 2017 Oct 31;8(5):e00517-17. doi: 10.1128/mBio.00517-17. mBio. 2017. PMID: 29089424 Free PMC article. - Hypermutator Pseudomonas aeruginosa Exploits Multiple Genetic Pathways To Develop Multidrug Resistance during Long-Term Infections in the Airways of Cystic Fibrosis Patients.
Colque CA, Albarracín Orio AG, Feliziani S, Marvig RL, Tobares AR, Johansen HK, Molin S, Smania AM. Colque CA, et al. Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02142-19. doi: 10.1128/AAC.02142-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32071060 Free PMC article. - Parallel evolution and local differentiation in quinolone resistance in Pseudomonas aeruginosa.
Wong A, Kassen R. Wong A, et al. Microbiology (Reading). 2011 Apr;157(Pt 4):937-944. doi: 10.1099/mic.0.046870-0. Epub 2011 Feb 3. Microbiology (Reading). 2011. PMID: 21292748 Review. - Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis.
Evans TJ. Evans TJ. Future Microbiol. 2015;10(2):231-9. doi: 10.2217/fmb.14.107. Future Microbiol. 2015. PMID: 25689535 Review.
Cited by
- Mutations causing low level antibiotic resistance ensure bacterial survival in antibiotic-treated hosts.
Frimodt-Møller J, Rossi E, Haagensen JAJ, Falcone M, Molin S, Johansen HK. Frimodt-Møller J, et al. Sci Rep. 2018 Aug 21;8(1):12512. doi: 10.1038/s41598-018-30972-y. Sci Rep. 2018. PMID: 30131514 Free PMC article. - Plasticity and Stereotypic Rewiring of the Transcriptome Upon Bacterial Evolution of Antibiotic Resistance.
Grézal G, Spohn R, Méhi O, Dunai A, Lázár V, Bálint B, Nagy I, Pál C, Papp B. Grézal G, et al. Mol Biol Evol. 2023 Feb 3;40(2):msad020. doi: 10.1093/molbev/msad020. Mol Biol Evol. 2023. PMID: 36718533 Free PMC article. - Burkholderia multivorans Exhibits Antibiotic Collateral Sensitivity.
Flanagan JN, Kavanaugh L, Steck TR. Flanagan JN, et al. Microb Drug Resist. 2020 Jan;26(1):1-8. doi: 10.1089/mdr.2019.0202. Epub 2019 Aug 8. Microb Drug Resist. 2020. PMID: 31393205 Free PMC article. - The PitA protein contributes to colistin susceptibility in Pseudomonas aeruginosa.
Erdmann MB, Gardner PP, Lamont IL. Erdmann MB, et al. PLoS One. 2023 Oct 12;18(10):e0292818. doi: 10.1371/journal.pone.0292818. eCollection 2023. PLoS One. 2023. PMID: 37824582 Free PMC article. - High-throughput laboratory evolution reveals evolutionary constraints in Escherichia coli.
Maeda T, Iwasawa J, Kotani H, Sakata N, Kawada M, Horinouchi T, Sakai A, Tanabe K, Furusawa C. Maeda T, et al. Nat Commun. 2020 Nov 24;11(1):5970. doi: 10.1038/s41467-020-19713-w. Nat Commun. 2020. PMID: 33235191 Free PMC article.
References
- Bantscheff M., Schirle M., Sweetman G., Rick J., Kuster B. Quantitative mass spectrometry in proteomics: A critical review. Anal. Bioanal. Chem. 2007;389:1017–1031. - PubMed
- Bonde M.T., Pedersen M., Klausen M.S., Jensen S.I., Wulff T., Harrison S., Nielsen A.T., Herrgård M.J., Sommer M.O.A. Predictable tuning of protein expression in bacteria. Nat. Methods. 2016;13:233–236. - PubMed
- Boucher H.W., Talbot G.H., Benjamin D.K., Jr., Bradley J., Guidos R.J., Jones R.N., Murray B.E., Bonomo R.A., Gilbert D., Infectious Diseases Society of America 10 x ’20 Progress—Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013;56:1685–1694. - PMC - PubMed
- Cabot G., Ocampo-Sosa A.A., Domínguez M.A., Gago J.F., Juan C., Tubau F., Rodríguez C., Moyà B., Peña C., Martínez-Martínez L., Oliver A., Spanish Network for Research in Infectious Diseases (REIPI) Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 2012;56:6349–6357. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical