Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase - PubMed (original) (raw)
Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase
Yuji Oe et al. Sci Rep. 2018.
Abstract
Liver dysfunction is a major problem in patients with severe preeclampsia (PE), hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, or in patients receiving anti-vascular endothelial growth factor (VEGF) therapy. Excessive soluble fms-like tyrosine kinase 1 (sFlt1) that antagonizes VEGF has been implicated in the pathogenesis of PE. VEGF increases the expression of endothelial nitric oxide synthase (eNOS) and activates it. eNOS polymorphisms that cause reduced NO production are associated with PE. The aim of this study was to clarify the role on hepatic function by excess sFlt1 in the absence of eNOS gene product. We first overexpressed sFlt1 using adenovirus in eNOS -/- and eNOS +/+ mice. Excessive sFlt1 and lack of eNOS synergistically increased plasma levels of liver transaminases, exacerbated infiltration of inflammatory cells, elevated expression levels of cytokines in the liver, and aggravated oxidative stress and coagulation abnormalities. Lack of eNOS in the presence of excess sFlt1 also induced thrombocytopenia, whereas eNOS +/+ mice with excess sFlt1 alone showed no or modest liver phenotype. Taken together, excessive sFlt1 and lack of eNOS synergistically induce hepatic dysfunction and thrombocytopenia, suggesting a novel role for VEGF and nitric oxide signaling in hepatocyte-endothelial cross-talk in health and in liver injury states.
Conflict of interest statement
S.A.K. is a coinventor of several patents related to angiogenic biomarkers that are held by BIDMC. S.A.K. reports serving as a consultant to Thermofisher Scientific, and has financial interest in Aggamin LLC.
Figures
Figure 1
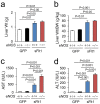
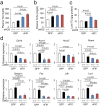
Liver dysfunction induced by excessive sFlt1 in mice lacking eNOS. (a) Liver weight (Wt). (b) Liver Wt/body weight (BW). Excessive sFlt1 causes hepatomegaly. The levels of plasma Aspartate transaminase (AST) and Alanine transaminase (ALT) are severely increased in eNOS −/−; sFlt1 mice (c,d) n = 7–9. Data are shown as mean ± s.e.m. ANOVA or Kruskal-Wallis test.
Figure 2
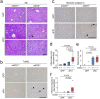
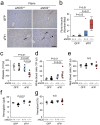
Histological damage in the liver. (a) Representative photomicrographs of Hematoxylin Eosin (HE). Inflammatory foci (arrowheads), vacuolar degeneration, and necrosis are shown in the liver from eNOS −/−; sFlt1 mice. TUNEL (b) and immunohistochemistry against cleaved caspase 3 (c) in the liver. Scale bar indicates 100 µm. (d) The number of inflammatory foci is significantly increased in the liver from eNOS −/−; sFlt1 mice. (e) The score of ballooning hepatocytes. (f) Increased cleaved caspase 3 positive cells in the liver from eNOS −/−; sFlt1 mice. n = 5–8. Data are shown as box plot. ANOVA or Kruskal-Wallis test.
Figure 3
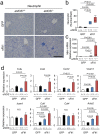
Inflammation in the liver. (a,b) Infiltrating neutrophils are visualized by Naphthol AS-D chloroacetate Esterase stain (blue). Scale bar indicates 100 µm. Number of neutrophil is increased by excessive sFlt1, which is further up-regulated by eNOS deletion. (c) The level of Mpo (myeloperoxidase) mRNA drastically increased in the liver from eNOS −/−; sFlt1 mice. (d) Expression of inflammation and pro-fibrotic related genes in the liver. N.S., not significant. n = 7–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Figure 4
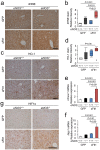
Markers of oxidative stress and hypoxia in the liver. (a) Representative photomicrographs of immunohistochemistry against 4-hydroxy-2-nonenal (4HNE). (b) Strong immunoreactive 4HNE is shown in the liver from eNOS −/−; sFlt1 mice. (c) Representative photomicrographs of immunohistochemistry against HO-1. (d) Strong immunoreactive HO-1 is shown in the liver from eNOS −/−; sFlt1 mice. (e,f) The levels of Hmox1 and Nqo1 mRNA in the liver. (g) Representative photomicrographs of immunohistochemistry against Hypoxia inducible factor 1α (HIF1α) in the liver. n = 5–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Figure 5
Lipid metabolism in the liver. (a–c) Plasma triglyceride (TG), total cholesterol (TCho), and hepatic TG. (d) Expression of Fatty acid oxidation, lipogenesis, and lipoprotein clearance receptor related genes in the liver, which are down-regulated by excessive sFlt1. n = 7–8. Data are shown as mean ± s.e.m. ANOVA or Kruskal-Wallis test.
Figure 6
Fibrin deposition in the liver and thrombocytopenia. (a) Representative photomicrographs of immunohistochemistry against Fibrin. Scale bar indicates 100 µm. (b) Number of Fibrin thrombi is significantly increased in the liver from eNOS −/−; sFlt1 mice. (c–g) Data of blood count; platelets (c), white blood cells (d), red blood cells (e), hemoglobin (f), and hematocrit (g). Excessive sFlt1 combined eNOS deletion causes thrombocytopenia. N.S., not significant. n = 4–8. Data are shown as mean ± s.e.m or box plot. ANOVA or Kruskal-Wallis test.
Similar articles
- Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia.
Burke SD, Zsengellér ZK, Khankin EV, Lo AS, Rajakumar A, DuPont JJ, McCurley A, Moss ME, Zhang D, Clark CD, Wang A, Seely EW, Kang PM, Stillman IE, Jaffe IZ, Karumanchi SA. Burke SD, et al. J Clin Invest. 2016 Jul 1;126(7):2561-74. doi: 10.1172/JCI83918. Epub 2016 Jun 6. J Clin Invest. 2016. PMID: 27270170 Free PMC article. - Thymoquinone mitigate ischemia-reperfusion-induced liver injury in rats: a pivotal role of nitric oxide signaling pathway.
Abd-Elbaset M, Arafa EA, El Sherbiny GA, Abdel-Bakky MS, Elgendy AN. Abd-Elbaset M, et al. Naunyn Schmiedebergs Arch Pharmacol. 2017 Jan;390(1):69-76. doi: 10.1007/s00210-016-1306-7. Epub 2016 Oct 7. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27717985 - eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype.
Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. Li F, et al. J Am Soc Nephrol. 2012 Apr;23(4):652-60. doi: 10.1681/ASN.2011040369. Epub 2012 Jan 26. J Am Soc Nephrol. 2012. PMID: 22282588 Free PMC article. - New progress in roles of nitric oxide during hepatic ischemia reperfusion injury.
Zhang YQ, Ding N, Zeng YF, Xiang YY, Yang MW, Hong FF, Yang SL. Zhang YQ, et al. World J Gastroenterol. 2017 Apr 14;23(14):2505-2510. doi: 10.3748/wjg.v23.i14.2505. World J Gastroenterol. 2017. PMID: 28465634 Free PMC article. Review. - Coagulation, Protease-Activated Receptors, and Diabetic Kidney Disease: Lessons from eNOS-Deficient Mice.
Oe Y, Miyazaki M, Takahashi N. Oe Y, et al. Tohoku J Exp Med. 2021 Sep;255(1):1-8. doi: 10.1620/tjem.255.1. Tohoku J Exp Med. 2021. PMID: 34511578 Review.
Cited by
- Ventilatory responses during and following hypercapnic gas challenge are impaired in male but not female endothelial NOS knock-out mice.
Getsy PM, Sundararajan S, May WJ, von Schill GC, McLaughlin DK, Palmer LA, Lewis SJ. Getsy PM, et al. Sci Rep. 2021 Oct 18;11(1):20557. doi: 10.1038/s41598-021-99922-5. Sci Rep. 2021. PMID: 34663876 Free PMC article. - The Impact of Coexisting Gestational Diabetes Mellitus on the Course of Preeclampsia.
Pankiewicz K, Szczerba E, Fijałkowska A, Sierdziński J, Issat T, Maciejewski TM. Pankiewicz K, et al. J Clin Med. 2022 Oct 28;11(21):6390. doi: 10.3390/jcm11216390. J Clin Med. 2022. PMID: 36362618 Free PMC article. - Protease-activated receptor 2 protects against VEGF inhibitor-induced glomerular endothelial and podocyte injury.
Oe Y, Fushima T, Sato E, Sekimoto A, Kisu K, Sato H, Sugawara J, Ito S, Takahashi N. Oe Y, et al. Sci Rep. 2019 Feb 27;9(1):2986. doi: 10.1038/s41598-019-39914-8. Sci Rep. 2019. PMID: 30814628 Free PMC article. - Oxidative Stress and Preeclampsia-Associated Prothrombotic State.
Han C, Huang P, Lyu M, Dong J. Han C, et al. Antioxidants (Basel). 2020 Nov 17;9(11):1139. doi: 10.3390/antiox9111139. Antioxidants (Basel). 2020. PMID: 33212799 Free PMC article. Review. - Placental Ischemia Says "NO" to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia.
Palei AC, Granger JP, Spradley FT. Palei AC, et al. Int J Mol Sci. 2021 Oct 19;22(20):11261. doi: 10.3390/ijms222011261. Int J Mol Sci. 2021. PMID: 34681920 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous