Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan - PubMed (original) (raw)
Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan
Yung-Yu Hsieh et al. Sci Rep. 2018.
Abstract
Helicobacter pylori is recognised as a main risk factor for gastric cancer. However, approximately half of the patients with gastritis are negative for H. pylori infection, and the abundance of H. pylori decreases in patients with cancer. In the current study, we profiled gastric epithelium-associated bacterial species in patients with gastritis, intestinal metaplasia, and gastric cancer to identify additional potential pathogenic bacteria. The overall composition of the microbiota was similar between the patients with gastritis and those with intestinal metaplasia. H. pylori was present in half of the non-cancer group, and the dominant bacterial species in the H. pylori-negative patients were Burkholderia, Enterobacter, and Leclercia. The abundance of those bacteria was similar between the cancer and non-cancer groups, whereas the frequency and abundance of H. pylori were significantly lower in the cancer group. Instead, Clostridium, Fusobacterium, and Lactobacillus species were frequently abundant in patients with gastric cancer, demonstrating a gastric cancer-specific bacterial signature. A receiver operating characteristic curve analysis showed that Clostridium colicanis and Fusobacterium nucleatum exhibited a diagnostic ability for gastric cancer. Our findings indicate that the gastric microenvironment is frequently colonised by Clostridium and Fusobacterium in patients with gastric cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
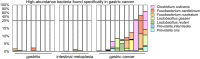
Identification of changes in the composition of the microbiota between patients with gastric cancer and those with other gastrointestinal disorders by 16S ribosomal DNA metagenomic analysis. (A) The abundance of Helicobacter pylori in the patients’ microbiota. (B) The proportions of high-abundance in-transit microbes. (C) The abundance of Burkholderia, Enterobacter, and Leclercia. (D) The abundance of Clostridium, Fusobacterium, and Lactobacillus.
Figure 2
Bacteria specifically enriched in the gastric cancer-associated microbiota.
Figure 3
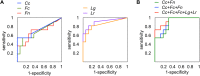
Identification of the bacterial signature of gastric cancer. Multidimensional analyses were performed considering various combinations of the following bacterial species: Clostridium colicanis (Cc), Fusobacterium canifelinum (Fc), Fusobacterium nucleatum (Fn), Lactobacillus gasseri (Lg), Lactobacillus reuteri (Lr), Megasphaera micronuciformis (Mm), Prevotella intermedia (Pi), Prevotella oris (Po), Streptococcus gordonii (Sg), and Streptococcus parasanguinis (Sp).
Figure 4
Evaluation of the discriminatory power of using bacterial abundance as a diagnostic tool. (A) The discriminatory power of the abundance of each individual species C. colicanis, F. canifelinum, F. nucleatum, L. gasseri, and L. reuteri was tested by a receiver operating characteristic curve analysis. (B) The combined abundance of C. colicanis and F. canifelinum (Cc + Fn), C. colicanis, F. canifelinum, and F. nucleatum (Cc + Fc + Fn), and C. colicanis, F. canifelinum, F. nucleatum, Lactobacillus gasseri, and Lactobacillus reuteri (Cc + Fc + Fn + Lg + Lr) was examined.
Similar articles
- Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods.
Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, Song KS, Kim YS, Kim JF. Eun CS, et al. Helicobacter. 2014 Dec;19(6):407-16. doi: 10.1111/hel.12145. Epub 2014 Jul 23. Helicobacter. 2014. PMID: 25052961 - Fusobacterium nucleatum is associated with worse prognosis in Lauren's diffuse type gastric cancer patients.
Boehm ET, Thon C, Kupcinskas J, Steponaitiene R, Skieceviciene J, Canbay A, Malfertheiner P, Link A. Boehm ET, et al. Sci Rep. 2020 Oct 1;10(1):16240. doi: 10.1038/s41598-020-73448-8. Sci Rep. 2020. PMID: 33004953 Free PMC article. - Association of Helicobacter pylori with carcinoma of stomach.
Arif M, Syed S. Arif M, et al. J Pak Med Assoc. 2007 Jul;57(7):337-41. J Pak Med Assoc. 2007. PMID: 17867254 - Fusobacterium nucleatum: Unraveling its potential role in gastric carcinogenesis.
Petkevicius V, Lehr K, Kupcinskas J, Link A. Petkevicius V, et al. World J Gastroenterol. 2024 Sep 21;30(35):3972-3984. doi: 10.3748/wjg.v30.i35.3972. World J Gastroenterol. 2024. PMID: 39351058 Free PMC article. Review. - Role of Helicobacter pylori gastritis in gastric atrophy, intestinal metaplasia, and gastric neoplasia.
Smith VC, Genta RM. Smith VC, et al. Microsc Res Tech. 2000 Mar 15;48(6):313-20. doi: 10.1002/(SICI)1097-0029(20000315)48:6<313::AID-JEMT1>3.0.CO;2-Y. Microsc Res Tech. 2000. PMID: 10738312 Review.
Cited by
- Clostridioides difficile antibody response of colorectal cancer patients versus clinically healthy individuals.
Magat EM, Balanag GA, CariÑo AM, Fellizar A, Ortin TS, Guevarra L Jr, Albano PM. Magat EM, et al. Biosci Microbiota Food Health. 2020;39(3):123-127. doi: 10.12938/bmfh.2020-010. Epub 2020 Feb 22. Biosci Microbiota Food Health. 2020. PMID: 32775130 Free PMC article. - The interplay between Helicobacter pylori and gastrointestinal microbiota.
Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. Chen CC, et al. Gut Microbes. 2021 Jan-Dec;13(1):1-22. doi: 10.1080/19490976.2021.1909459. Gut Microbes. 2021. PMID: 33938378 Free PMC article. Review. - Network construction of gastric microbiome and organization of microbial modules associated with gastric carcinogenesis.
Park CH, Lee JG, Lee AR, Eun CS, Han DS. Park CH, et al. Sci Rep. 2019 Aug 27;9(1):12444. doi: 10.1038/s41598-019-48925-4. Sci Rep. 2019. PMID: 31455798 Free PMC article. - Suicide journey of H. pylori through gastric carcinogenesis: the role of non-H. pylori microbiome and potential consequences for clinical practice.
de Assumpção PP, Araújo TMT, de Assumpção PB, Barra WF, Khayat AS, Assumpção CB, Ishak G, Nunes DN, Dias-Neto E, Coelho LGV. de Assumpção PP, et al. Eur J Clin Microbiol Infect Dis. 2019 Sep;38(9):1591-1597. doi: 10.1007/s10096-019-03564-5. Epub 2019 Apr 17. Eur J Clin Microbiol Infect Dis. 2019. PMID: 31114971 Review. - Inhibition of EZH2 and activation of ERRγ synergistically suppresses gastric cancer by inhibiting FOXM1 signaling pathway.
Huang B, Mu P, Yu Y, Zhu W, Jiang T, Deng R, Feng G, Wen J, Zhu X, Deng Y. Huang B, et al. Gastric Cancer. 2021 Jan;24(1):72-84. doi: 10.1007/s10120-020-01097-x. Epub 2020 Jun 11. Gastric Cancer. 2021. PMID: 32529327
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical