Functional Implications of Intracellular Phase Transitions - PubMed (original) (raw)
Functional Implications of Intracellular Phase Transitions
Alex S Holehouse et al. Biochemistry. 2018.
Abstract
Intracellular environments are heterogeneous milieus comprised of macromolecules, osmolytes, and a range of assemblies that include membrane-bound organelles and membraneless biomolecular condensates. The latter are nonstoichiometric assemblies of protein and RNA molecules. They represent distinct phases and form via intracellular phase transitions. Here, we present insights from recent studies and provide a perspective on how phase transitions that lead to biomolecular condensates might contribute to cellular functions.
Figures
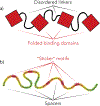
Figure 1.. Schematic showing how multivalency could be manifest in biomacromolecules.
a) Linear multivalent proteins with folded binding domains connected by flexible intrinsically disordered linkers. A similar polymer architecture could in principle also be formed by an RNA molecule. b) A fully disordered polymer (disordered protein region or an RNA molecule) composed of regions that mediate intermolecular interactions (stickers) and flexible non-adhesive spacers. The relative proportion of sticker and spacer, the strength of sticker-mediated interactions, and the intrinsic dynamics of spacers, will all contribute to material properties of a condensate.
Figure 2.. Summary of a subset of putative mechanistic and regulatory features that phase transitions might support.
For convenience, all phase diagrams drawn here are in the inverse χ (χ−1) versus concentration plane, but the ordinate could alternatively be one of any number of parameters such as salt concentration, pH, concentrations of binding partners etc. The parameter χ−1 provides a measure of the strength of interaction between constitutive components (high values reflect weaker interactions). These diagrams are meant to be illustrative of the types of phenomena one might expect to see. a) Condensates facilitate a high local concentration of scaffold and client components. b) Through the partitioning of distinct components, the condensate interior can provide a specific repertoire of macromolecules. c) A phase boundary provides a passive mechanism to fix the bulk concentration of scaffold components. At concentrations above the low concentration arm of the coexistence line, additional components partition into condensates, while the concentration in the soluble phase remains unchanged. In this way, the volumes of the two phases will change, but their concentrations will not. d) The low concentration arm of the coexistence curve can move right (top) or left (bottom), leading to an increase or decrease in the saturation concentration, respectively. e) The critical point can move up or down, such that for a system where the position on the ordinate was already close to the critical temperature a shifting of the critical point down could eliminate the ability to form condensates (bottom). f) The high concentration arm of the coexistence curve can move left (top) or right (bottom) indicating a decrease or an increase in the concentration of components inside the condensate, respectively. g) For multicomponent assemblies, the presence or absence of a single component can trigger the formation of an arbitrarily complex assembly with multi-phase behaviour. h) Condensates with similar components may have very different functional outputs in response to the presence of one specific component versus another. i) Condensate dissolution and disassembly dynamics may be complex and could depend on multiple different factors, leading to rates that are quite different to those predicted by simple mean-field models. A condensate could persist for long timescales even after the bulk concentration is below the saturation concentration if the droplet was kinetically trapped (top row). If the components contain multiple distinct interacting domains, once condensate formation has been driven by one set of domains, additional interactions may now occur via an orthogonal mechanism (middle row). A secondary nucleation-dependent process (such as solid formation, e.g. spherulite or amyloid growth, as depicted in the bottom row) could occur within the condensate, allowing a liquid-like droplet to transition into an alternative state according to some characteristic and sequence/composition dependent timescale.
Similar articles
- Analysis of biomolecular condensates and protein phase separation with microfluidic technology.
Linsenmeier M, Kopp MRG, Stavrakis S, de Mello A, Arosio P. Linsenmeier M, et al. Biochim Biophys Acta Mol Cell Res. 2021 Jan;1868(1):118823. doi: 10.1016/j.bbamcr.2020.118823. Epub 2020 Aug 13. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 32800925 Review. - Optogenetic Reconstitution for Determining the Form and Function of Membraneless Organelles.
Dine E, Toettcher JE. Dine E, et al. Biochemistry. 2018 May 1;57(17):2432-2436. doi: 10.1021/acs.biochem.7b01173. Epub 2018 Jan 26. Biochemistry. 2018. PMID: 29373016 Free PMC article. - Reentrant Phase Transitions and Non-Equilibrium Dynamics in Membraneless Organelles.
Milin AN, Deniz AA. Milin AN, et al. Biochemistry. 2018 May 1;57(17):2470-2477. doi: 10.1021/acs.biochem.8b00001. Epub 2018 Apr 3. Biochemistry. 2018. PMID: 29569441 Free PMC article. - A guide to regulation of the formation of biomolecular condensates.
Bratek-Skicki A, Pancsa R, Meszaros B, Van Lindt J, Tompa P. Bratek-Skicki A, et al. FEBS J. 2020 May;287(10):1924-1935. doi: 10.1111/febs.15254. Epub 2020 Mar 14. FEBS J. 2020. PMID: 32080961 Review. - Biomolecular condensates in cell biology and virology: Phase-separated membraneless organelles (MLOs).
Sehgal PB, Westley J, Lerea KM, DiSenso-Browne S, Etlinger JD. Sehgal PB, et al. Anal Biochem. 2020 May 15;597:113691. doi: 10.1016/j.ab.2020.113691. Epub 2020 Mar 16. Anal Biochem. 2020. PMID: 32194074 Review.
Cited by
- Reduced dynamicity and increased high-order protein assemblies in dense fibrillar component of the nucleolus under cellular senescence.
Jo M, Kim S, Park J, Chang YT, Gwon Y. Jo M, et al. Redox Biol. 2024 Sep;75:103279. doi: 10.1016/j.redox.2024.103279. Epub 2024 Jul 25. Redox Biol. 2024. PMID: 39111063 Free PMC article. - De novo engineering of intracellular condensates using artificial disordered proteins.
Dzuricky M, Rogers BA, Shahid A, Cremer PS, Chilkoti A. Dzuricky M, et al. Nat Chem. 2020 Sep;12(9):814-825. doi: 10.1038/s41557-020-0511-7. Epub 2020 Aug 3. Nat Chem. 2020. PMID: 32747754 Free PMC article. - Multiphase Complex Coacervate Droplets.
Lu T, Spruijt E. Lu T, et al. J Am Chem Soc. 2020 Feb 12;142(6):2905-2914. doi: 10.1021/jacs.9b11468. Epub 2020 Jan 30. J Am Chem Soc. 2020. PMID: 31958956 Free PMC article. - Coactivator condensation drives cardiovascular cell lineage specification.
Gan P, Eppert M, De La Cruz N, Lyons H, Shah AM, Veettil RT, Chen K, Pradhan P, Bezprozvannaya S, Xu L, Liu N, Olson EN, Sabari BR. Gan P, et al. Sci Adv. 2024 Mar 15;10(11):eadk7160. doi: 10.1126/sciadv.adk7160. Epub 2024 Mar 15. Sci Adv. 2024. PMID: 38489358 Free PMC article. - A Few Charged Residues in Galectin-3's Folded and Disordered Regions Regulate Phase Separation.
Sun YC, Hsieh TL, Lin CI, Shao WY, Lin YH, Huang JR. Sun YC, et al. Adv Sci (Weinh). 2024 Nov;11(41):e2402570. doi: 10.1002/advs.202402570. Epub 2024 Sep 9. Adv Sci (Weinh). 2024. PMID: 39248370 Free PMC article.
References
- Skibinski G, and Finkbeiner S (2013) Longitudinal measures of proteostasis in live neurons: features that determine fate in models of neurodegenerative disease, FEBS letters 587, 1139–1146. - PubMed
- Shin Y, and Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease, Science 357, eaaf4382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources