mRNA vaccines - a new era in vaccinology - PubMed (original) (raw)
Review
mRNA vaccines - a new era in vaccinology
Norbert Pardi et al. Nat Rev Drug Discov. 2018 Apr.
Abstract
mRNA vaccines represent a promising alternative to conventional vaccine approaches because of their high potency, capacity for rapid development and potential for low-cost manufacture and safe administration. However, their application has until recently been restricted by the instability and inefficient in vivo delivery of mRNA. Recent technological advances have now largely overcome these issues, and multiple mRNA vaccine platforms against infectious diseases and several types of cancer have demonstrated encouraging results in both animal models and humans. This Review provides a detailed overview of mRNA vaccines and considers future directions and challenges in advancing this promising vaccine platform to widespread therapeutic use.
Conflict of interest statement
Competing interests statement
The authors declare competing interests: see Web version for details.
Figures
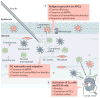
Figure 1. Innate immune sensing of mRNA vaccines
Innate immune sensing of two types of mRNA vaccine by a dendritic cell (DC), with RNA sensors shown in yellow, antigen in red, DC maturation factors in green, and peptide–major histocompatibility complex (MHC) complexes in light blue and red; an example lipid nanoparticle carrier is shown at the top right. A non-exhaustive list of the major known RNA sensors that contribute to the recognition of double-stranded and unmodified single-stranded RNAs is shown. Unmodified, unpurified (part a) and nucleoside-modified, fast protein liquid chromatography (FPLC)-purified (part b) mRNAs were selected for illustration of two formats of mRNA vaccines where known forms of mRNA sensing are present and absent, respectively. The dashed arrow represents reduced antigen expression. Ag, antigen; PKR, interferon-induced, double-stranded RNA-activated protein kinase; MDA5, interferon-induced helicase C domain-containing protein 1 (also known as IFIH1); IFN, interferon; m1Ψ, 1-methylpseudouridine; OAS, 2′–5′-oligoadenylate synthetase; TLR, Toll-like receptor.
Figure 2. Major delivery methods for mRNA vaccines
Commonly used delivery methods and carrier molecules for mRNA vaccines along with typical diameters for particulate complexes are shown: naked mRNA (part a); naked mRNA with in vivo electroporation (part b); protamine (cationic peptide)-complexed mRNA (part c); mRNA associated with a positively charged oil-in-water cationic nanoemulsion (part d); mRNA associated with a chemically modified dendrimer and complexed with polyethylene glycol (PEG)-lipid (part e); protamine-complexed mRNA in a PEG-lipid nanoparticle (part f); mRNA associated with a cationic polymer such as polyethylenimine (PEI) (part g); mRNA associated with a cationic polymer such as PEI and a lipid component (part h); mRNA associated with a polysaccharide (for example, chitosan) particle or gel (part i); mRNA in a cationic lipid nanoparticle (for example, 1,2-dioleoyloxy-3-trimethylammoniumpropane (DOTAP) or dioleoylphosphatidylethanolamine (DOPE) lipids) (part j); mRNA complexed with cationic lipids and cholesterol (part k); and mRNA complexed with cationic lipids, cholesterol and PEG-lipid (part l).
Figure 3. Considerations for effectiveness of a directly injected mRNA vaccine
For an injected mRNA vaccine, major considerations for effectiveness include the following: the level of antigen expression in professional antigen-presenting cells (APCs), which is influenced by the efficiency of the carrier, by the presence of pathogen-associated molecular patterns (PAMPs) in the form of double-stranded RNA (dsRNA) or unmodified nucleosides and by the level of optimization of the RNA sequence (codon usage, G:C content, 5′ and 3′ untranslated regions (UTRs) and so on); dendritic cell (DC) maturation and migration to secondary lymphoid tissue, which is increased by PAMPs; and the ability of the vaccine to activate robust T follicular helper (TFH) cell and germinal centre (GC) B cell responses — an area that remains poorly understood. An intradermal injection is shown as an example. EC, extracellular.
Similar articles
- mRNA vaccine for cancer immunotherapy.
Miao L, Zhang Y, Huang L. Miao L, et al. Mol Cancer. 2021 Feb 25;20(1):41. doi: 10.1186/s12943-021-01335-5. Mol Cancer. 2021. PMID: 33632261 Free PMC article. Review. - The use of RNA-based treatments in the field of cancer immunotherapy.
Chehelgerdi M, Chehelgerdi M. Chehelgerdi M, et al. Mol Cancer. 2023 Jul 7;22(1):106. doi: 10.1186/s12943-023-01807-w. Mol Cancer. 2023. PMID: 37420174 Free PMC article. Review. - Challenges and advances towards the rational design of mRNA vaccines.
Pollard C, De Koker S, Saelens X, Vanham G, Grooten J. Pollard C, et al. Trends Mol Med. 2013 Dec;19(12):705-13. doi: 10.1016/j.molmed.2013.09.002. Epub 2013 Oct 15. Trends Mol Med. 2013. PMID: 24138818 Review. - Recent advances in mRNA-based vaccine for cancer therapy; bench to bedside.
Kenoosh HA, Pallathadka H, Hjazi A, Al-Dhalimy AMB, Zearah SA, Ghildiyal P, Al-Mashhadani ZI, Mustafa YF, Hizam MM, Elawady A. Kenoosh HA, et al. Cell Biochem Funct. 2024 Mar;42(2):e3954. doi: 10.1002/cbf.3954. Cell Biochem Funct. 2024. PMID: 38403905 Review. - Recent advances in mRNA vaccine technology.
Pardi N, Hogan MJ, Weissman D. Pardi N, et al. Curr Opin Immunol. 2020 Aug;65:14-20. doi: 10.1016/j.coi.2020.01.008. Epub 2020 Mar 31. Curr Opin Immunol. 2020. PMID: 32244193 Review.
Cited by
- mRNA compartmentalization via multimodule DNA nanostructure assembly augments the immunogenicity and efficacy of cancer mRNA vaccine.
Guo X, Guo M, Cai R, Hu M, Rao L, Su W, Liu H, Gao F, Zhang X, Liu J, Chen C. Guo X, et al. Sci Adv. 2024 Nov 22;10(47):eadp3680. doi: 10.1126/sciadv.adp3680. Epub 2024 Nov 22. Sci Adv. 2024. PMID: 39576858 Free PMC article. - Safety, efficacy, and immunogenicity of SARS-CoV-2 mRNA vaccination in children and adult patients with rheumatic diseases: a comprehensive literature review.
Dhanasekaran P, Karasu BT, Mak A. Dhanasekaran P, et al. Rheumatol Int. 2024 Dec;44(12):2757-2794. doi: 10.1007/s00296-024-05734-x. Epub 2024 Nov 22. Rheumatol Int. 2024. PMID: 39576327 Review. - A convenient analytic method for gel quantification using ImageJ paired with Python or R.
Tomlinson C, Rajasekaran A, Brochu-Gaudreau K, Dubois C, Farmilo AJ, Gris P, Khatiz A, Matthews A, Piltonen M, Amrani A, Gris D. Tomlinson C, et al. PLoS One. 2024 Nov 21;19(11):e0308297. doi: 10.1371/journal.pone.0308297. eCollection 2024. PLoS One. 2024. PMID: 39570862 Free PMC article. - COVID-19 vaccines: current and future challenges.
Mohammadi D, Ghasemi M, Manouchehrian N, Zafarmand M, Akbari M, Boroumand AB. Mohammadi D, et al. Front Pharmacol. 2024 Nov 6;15:1434181. doi: 10.3389/fphar.2024.1434181. eCollection 2024. Front Pharmacol. 2024. PMID: 39568586 Free PMC article. Review. - Bioinformatics designing of an mRNA vaccine for Mokola virus (MOKV) using immunoinformatics as a secure strategy for successful vaccine development.
Oladipo EK, Ogunniran JA, Akinpelu OS, Omole TO, Adeyemo SF, Irewolede BA, Iwalokun BA, Ajani OF, Onyeaka H. Oladipo EK, et al. BMC Immunol. 2024 Nov 20;25(1):77. doi: 10.1186/s12865-024-00668-2. BMC Immunol. 2024. PMID: 39563241 Free PMC article.
References
- World Health Organization. Immunization coverage. World Health Organization; 2017. http://www.who.int/mediacentre/factsheets/fs378/en.
- Younger DS, Younger AP, Guttmacher S. Childhood vaccination: implications for global and domestic public health. Neurol Clin. 2016;34:1035–1047. - PubMed
- Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. This study demonstrates protein production from RNA administered in vivo. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI100645/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- R01 AI090788/AI/NIAID NIH HHS/United States
- R01 AI124429/AI/NIAID NIH HHS/United States
- R01 AI050484/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical