Control of directionality in lambda site specific recombination - PubMed (original) (raw)
Control of directionality in lambda site specific recombination
W Bushman et al. Science. 1985.
Abstract
The simple relation between the substrates and products of site-specific recombination raises questions about the control of directionality often observed in this class of DNA transactions. For bacteriophage lambda, viral integration and excision proceed by discrete pathways, and DNA substrates with the intrinsic property of recombining in only one direction can be constructed. These pathways display an asymmetric reliance on a complex array of protein binding sites, and they respond differently to changes in the concentrations of the relevant proteins. The Escherichia coli protein integration host factor (IHF) differentially affects integrative and excisive recombination, thereby influencing directionality. A four- to eightfold increase in intracellular IHF coincides with the transition from exponential to stationary phase; this provides a mechanism for growth phase-dependent regulation of recombination that makes the cellular physiology an intrinsic part of the recombination reaction.
Figures
Fig. 1
The DNA substrates, proteins, and protein binding sites in lambda site specific recombination: Circular phage DNA (_att_P,—), and linear bacterial DNA (_att_B,═) undergo integrative recombination to yield _att_L and _att_R. For _att_P and _att_B, the core-type Int sites and overlap regions (COC′ and BOB′, respectively) are indicated. For _att_L and _att_R, which can undergo excisive recombination to yield _att_P and _att_B, the top strand DNA sequence is shown (49). The protein binding sites for IHF (H), Xis (X), arm-type Int (P), and core-type Int (C or B) are numbered from left to right and marked with prime signs if present to the right of the overlap region. Although the regions underscored with symbols include all of the bases apparently important for specific protein recognition, some of the interior bases are not specified in the consensus sequences (8, 10,11,20, 50). The relative orientation of sites for a given protein is indicated by the symbols (IHF: H or H, arm-type Int: A or A core-type Int: c or ○, and Xis: ×▸). The sites of strand exchange in the top strand (↘) and omitted bottom strand (↖) demark the 7-bp overlap region (○). The resected _att_R sites (lower portion) are named for their outermost complete protein binding site as described in the text (Nde-B′ refers to an _att_R cut with Nde I prior to use in order to remove all DNA to the left of −99). The last base for which each resected _att_R matches the unresected att DNA is shown (↑). Most resected _att_R plasmids were constructed from _att_P plasmids by in vitro recombination. The construction of P1-P′3, P2-P′3, X2-P′3, H2-P′3, and C-P′3 has been described (18). H1-B′ was made by (i) digestion of P1-B′ with Dde I, (ii) filling in the staggered ends with the Klenow fragment of DNA polymerase, (iii) gel purification of the appropriate sized fragment on low-melting agarose, and then digestion with Bam HI. This was ligated to the large Eco RV-Bam HI fragment of pBR327. X1-B′ was made by cutting P1-B′ and pBR322 with Nde I and Bam HI. The appropriate fragments were then ligated. B-H′ (end point at +46), B-P′1 (+64), and B-P′3 (+100) were made by recombination in vitro with previously described resected _att_P plasmids (18). Constructions that are missing different extents of the same protein binding site are not shown separately because identical results were obtained within such sets.
Fig. 2
Excisive and integrative recombination with resected att sites. Excisive recombinations with supercoiled _att_R. and radioactive linear att_L; and integrative recombination, with supercoiled att_P and radioactive linear att_B, were carried out in vitro (24). The identity of the att_R and att_P substrates used in the reactions is indicated at the top of each lane. att_L and att_B were linearized with Eco RI and labeled with [α-32P]dATP and the Klenow fragment of DNA polymerase. Labeled DNA’s were 1.25 × 10−11_M in the 20-μl sample. Purified proteins were added to the DNA-buffer mix in the following order and concentrations (for excision and integration, respectively): IHF (2.5 n_M and 10 n_M), Int (62 n_M and 125 n_M), and Xis (150 n_M and 0 n_M). After 4 hours at 25°C, 10 μl of "loading" solution [1 percent SDS, 10 percent Ficoll, salmon sperm DNA (25 μg/ml)] were added to each sample and placed onto a 1.2 percent agarose gel. After electrophoresis for 16 hours at 40 V, the gels were dried and autoradiographed. The product for the reaction of H1-B′ with _att_L is smaller than for reactions involving the other _att_R DNA’s because it is on the smaller pBR327 backbone rather than pBR322.
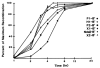
Fig. 3
Kinetics of excisive recombination: Labeled att_R substrates were recombined with supercoiled att_L as described in Fig. 2, except that IHF, Int, and Xis were present at 1.25 n_M, 25 n_M, and 150 n_M_, respectively, and reactions were done at 20°C so that kinetics could be more accurately studied. Similar results are obtained at 25°C. Autoradiograms were analyzed with a Hoefer GS300 scanning densitometer attached to a Hewlett-Packard 3390A integrator. Percentages of recombination for each time point were normalized to the percentage obtained at 24 hours for each combination of att sites. The values for the efficiency at 24 hours were 39 percent (P1-B′), 41 percent (H1-B′), 43 percent (P2-B′), 48 percent (X1-B′), 25 percent (Nde-B′), and 12 percent (X2-B′).
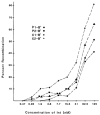
Fig. 4
Int dependence of excision. The concentration of Int was varied in twofold increments under the recombination conditions described in Fig; 3, except that IHF and Xis were present at 5 n_M_ and 150 n_M_, respectively. The abscissa is a logarithmic scale and the ordinate is the absolute percentage of each _att_R recombined (not the fraction of the final recombination as in Fig. 3).
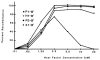
Fig. 5
Xis dependence of excision. The concentration of Xis was varied in twofold increments (and plotted on a logarithmic scale) under the recombination conditions described in Fig. 3. IHF and Int were 5 n_M_ and 60 n_M_, respectively.
Fig. 6
IHF dependence of excision. The concentration of IHF was varied in twofold increments (and plotted on a logarithmic scale) as described in Fig. 3. Int and Xis were 12 n_M_ and 150 n_M_, respectively.
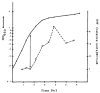
Fig. 7
Intracellular IHF as a function of growth phase. E. coli strain HN356 (9) was grown in rich medium, and optical density (OD) was measured at 650 nm. A double-headed arrow shows the last point during exponential phase growth. Cells (constant wet weight) were harvested (non-linearity of light scattering at high cell density was taken into account by diluting cells to determine the volume needed), centrifuged (SS34 rotor) at 4000 rev/min, and resuspended in 10 m_M_ tris (p_H 7.9) containing 1 m_M EDTA. Cells were lysed on ice by addition of NaOH to 0.1_M_ and vortexed for 1 minute. The cells were put on ice for 15 minutes and HCl was added to 0.2_M_; they were then vortexed for 1 minute. After sedimenting the cellular debris in a microfuge, the supernatant was neutralized with tris base and assayed for DNA binding activity in a solution with 5 × 10−13_M_ labeled DNA containing IHF binding sites, unlabeled salmon sperm DNA (100 μg/ml), 100 m_M_ NaCl, 10 m_M_ MgCl2, and 50 m_M_ tris (_p_H 7.9). These samples were then subjected to electrophoresis on 5 percent polyacrylamide gels as described (24). The DNA binding patterns of the in vivo extracts were compared to samples containing known amounts of IHF. With this method, the yield of IHF from exponential phase cells is approximately the same as that obtained in the original IHF purification (9). The ratio between IHF present in the stationary (as compared to the exponential phase) varied between 3 and 10 in different experiments.
Similar articles
- Mutations in an integration host factor-binding site: effect on lambda site-specific recombination and regulatory implications.
Thompson JF, Waechter-Brulla D, Gumport RI, Gardner JF, Moitoso de Vargas L, Landy A. Thompson JF, et al. J Bacteriol. 1986 Dec;168(3):1343-51. doi: 10.1128/jb.168.3.1343-1351.1986. J Bacteriol. 1986. PMID: 2946666 Free PMC article. - Bacteriophage lambda site-specific recombination.
Van Duyne GD, Landy A. Van Duyne GD, et al. Mol Microbiol. 2024 May;121(5):895-911. doi: 10.1111/mmi.15241. Epub 2024 Feb 19. Mol Microbiol. 2024. PMID: 38372210 Free PMC article. Review. - Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination.
Nash HA. Nash HA. Annu Rev Genet. 1981;15:143-67. doi: 10.1146/annurev.ge.15.120181.001043. Annu Rev Genet. 1981. PMID: 6461289 Review. No abstract available. - Nucleoprotein architectures regulating the directionality of viral integration and excision.
Seah NE, Warren D, Tong W, Laxmikanthan G, Van Duyne GD, Landy A. Seah NE, et al. Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12372-7. doi: 10.1073/pnas.1413019111. Epub 2014 Aug 11. Proc Natl Acad Sci U S A. 2014. PMID: 25114241 Free PMC article. - Lambda site-specific recombination: the att site.
Gottesman S. Gottesman S. Cell. 1981 Sep;25(3):585-6. doi: 10.1016/0092-8674(81)90165-3. Cell. 1981. PMID: 6456816 No abstract available.
Cited by
- Structure of a Holliday junction complex reveals mechanisms governing a highly regulated DNA transaction.
Laxmikanthan G, Xu C, Brilot AF, Warren D, Steele L, Seah N, Tong W, Grigorieff N, Landy A, Van Duyne GD. Laxmikanthan G, et al. Elife. 2016 May 25;5:e14313. doi: 10.7554/eLife.14313. Elife. 2016. PMID: 27223329 Free PMC article. - Helical-repeat dependence of integrative recombination of bacteriophage lambda: role of the P1 and H1 protein binding sites.
Thompson JF, Snyder UK, Landy A. Thompson JF, et al. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6323-7. doi: 10.1073/pnas.85.17.6323. Proc Natl Acad Sci U S A. 1988. PMID: 2842765 Free PMC article. - Architecture of recombination intermediates visualized by in-gel FRET of lambda integrase-Holliday junction-arm DNA complexes.
Radman-Livaja M, Biswas T, Mierke D, Landy A. Radman-Livaja M, et al. Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):3913-20. doi: 10.1073/pnas.0500844102. Epub 2005 Mar 7. Proc Natl Acad Sci U S A. 2005. PMID: 15753294 Free PMC article. - Protein and DNA requirements of the bacteriophage HP1 recombination system: a model for intasome formation.
Esposito D, Thrower JS, Scocca JJ. Esposito D, et al. Nucleic Acids Res. 2001 Oct 1;29(19):3955-64. doi: 10.1093/nar/29.19.3955. Nucleic Acids Res. 2001. PMID: 11574677 Free PMC article. - A chimeric Cre recombinase with regulated directionality.
Warren D, Laxmikanthan G, Landy A. Warren D, et al. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18278-83. doi: 10.1073/pnas.0809949105. Epub 2008 Nov 14. Proc Natl Acad Sci U S A. 2008. PMID: 19011106 Free PMC article.
References
- Weisberg RA, Landy A. In: Lambda II. Hendrix R, Roberts J, Stahl F, Weisberg R, editors. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1983. p. 211.
- Nash HA. Annu Rev Genet. 1981;15:143. - PubMed
- Mizuuchi K, et al. Cold Spring Harbor Symp Quant Biol. 1981;45:429. - PubMed
- Bauer CE, Gardner JF, Gumport RI. J Mol Biol. 1985;181:187. - PubMed
- Holliday R. Genet Res. 1974;5:282.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources