Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study - PubMed (original) (raw)
Clinical Trial
Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study
John H Beigel et al. Lancet Infect Dis. 2018 Apr.
Abstract
Background: Middle East respiratory syndrome (MERS) is a severe respiratory illness with an overall mortality of 35%. There is no licensed or proven treatment. Passive immunotherapy approaches are being developed to prevent and treat several human medical conditions where alternative therapeutic options are absent. We report the safety of a fully human polyclonal IgG antibody (SAB-301) produced from the hyperimmune plasma of transchromosomic cattle immunised with a MERS coronavirus vaccine.
Methods: We did a phase 1 double-blind, placebo-controlled, single-dose escalation trial at the National Institutes of Health Clinical Center. We recruited healthy participants aged 18-60 years who had normal laboratory parameters at enrolment, a body-mass index of 19-32 kg/m2, and a creatinine clearance of 70 mL/min or more, and who did not have any chronic medical problems that required daily oral medications, a positive rheumatoid factor (≥15 IU/mL), IgA deficiency (<7 mg/dL), or history of allergy to intravenous immunoglobulin or human blood products. Participants were randomly assigned by a computer-generated table, made by a masked pharmacist, to one of six cohorts (containing between three and ten participants each). Cohorts 1 and 2 had three participants, randomly assigned 2:1 to receive active drug SAB-301 versus normal saline placebo; cohorts 3 and 4 had six participants randomised 2:1; and cohorts 5 and 6 had ten participants, randomised 4:1. Participants received 1 mg/kg, 2·5 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg, or 50 mg/kg of SAB-301, or equivalent volume placebo (saline control), on day 0, and were followed up by clinical, laboratory, and pharmacokinetic assessments on days 1, 3, 7, 21, 42, and 90. The primary outcome was safety, and immunogenicity was a secondary outcome. We analysed the intention-to-treat population. This trial is registered with ClinicalTrials.gov, number NCT02788188.
Findings: Between June 2, 2016, and Jan 4, 2017, we screened 43 participants, of whom 38 were eligible and randomly assigned to receive SAB-301 (n=28) or placebo (n=10). 97 adverse events were reported: 64 adverse events occurred in 23 (82%) of 28 participants receiving SAB-301 (mean 2·3 adverse events per participant). 33 adverse events occurred in all ten participants receiving placebo (mean 3·3 adverse events per participant). The most common adverse events were headache (n=6 [21%] in participants who received SAB-301 and n=2 [20%] in those receiving placebo), albuminuria (n=5 [18%] vs n=2 [20%]), myalgia (n=3 [11%] vs n=1 [10%]), increased creatine kinase (n=3 [11%] vs 1 [10%]), and common cold (n=3 [11%] vs n=2 [20%]). There was one serious adverse event (hospital admission for suicide attempt) in one participant who received 50 mg/kg of SAB-301. The area under the concentration-time curve (AUC) in the 50 mg/kg dose (27 498 μg × days per mL) is comparable to the AUC that was associated with efficacy in a preclinical model.
Interpretation: Single infusions of SAB-301 up to 50 mg/kg appear to be safe and well tolerated in healthy participants. Human immunoglobulin derived from transchromosomic cattle could offer a new platform technology to produce fully human polyclonal IgG antibodies for other medical conditions.
Funding: National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Biomedical Advanced Research and Development Authority.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
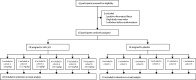
Figure 1
Trial profile
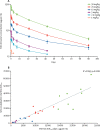
Figure 2
Pharmacokinetic analysis (A) Mean plasma concentrations of SAB-301 (μg/mL) over time in cohorts one to six. SEM shown as error bars. (B) SAB-301 AUC vs viral neutralisation titre AUC, in all cohorts. AUClast=area under the concentration–time curve from 0 h to the last pharmacokinetic sample post-dose.
Comment in
- Herd immunity: hyperimmune globulins for the 21st century.
Elliott ST, Weiner DB. Elliott ST, et al. Lancet Infect Dis. 2018 Apr;18(4):361-363. doi: 10.1016/S1473-3099(18)30003-3. Epub 2018 Jan 9. Lancet Infect Dis. 2018. PMID: 29329956 Free PMC article. No abstract available.
Similar articles
- Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study.
Heppner DG Jr, Kemp TL, Martin BK, Ramsey WJ, Nichols R, Dasen EJ, Link CJ, Das R, Xu ZJ, Sheldon EA, Nowak TA, Monath TP; V920-004 study team. Heppner DG Jr, et al. Lancet Infect Dis. 2017 Aug;17(8):854-866. doi: 10.1016/S1473-3099(17)30313-4. Epub 2017 Jun 9. Lancet Infect Dis. 2017. PMID: 28606591 Clinical Trial. - Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial.
Pollard AJ, Launay O, Lelievre JD, Lacabaratz C, Grande S, Goldstein N, Robinson C, Gaddah A, Bockstal V, Wiedemann A, Leyssen M, Luhn K, Richert L, Bétard C, Gibani MM, Clutterbuck EA, Snape MD, Levy Y, Douoguih M, Thiebaut R; EBOVAC2 EBL2001 study group. Pollard AJ, et al. Lancet Infect Dis. 2021 Apr;21(4):493-506. doi: 10.1016/S1473-3099(20)30476-X. Epub 2020 Nov 17. Lancet Infect Dis. 2021. PMID: 33217361 Clinical Trial. - Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial.
Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, Reuschel EL, Robb ML, Racine T, Oh MD, Lamarre C, Zaidi FI, Boyer J, Kudchodkar SB, Jeong M, Darden JM, Park YK, Scott PT, Remigio C, Parikh AP, Wise MC, Patel A, Duperret EK, Kim KY, Choi H, White S, Bagarazzi M, May JM, Kane D, Lee H, Kobinger G, Michael NL, Weiner DB, Thomas SJ, Maslow JN. Modjarrad K, et al. Lancet Infect Dis. 2019 Sep;19(9):1013-1022. doi: 10.1016/S1473-3099(19)30266-X. Epub 2019 Jul 24. Lancet Infect Dis. 2019. PMID: 31351922 Free PMC article. Clinical Trial. - Anti-influenza immune plasma for the treatment of patients with severe influenza A: a randomised, double-blind, phase 3 trial.
Beigel JH, Aga E, Elie-Turenne MC, Cho J, Tebas P, Clark CL, Metcalf JP, Ozment C, Raviprakash K, Beeler J, Holley HP Jr, Warner S, Chorley C, Lane HC, Hughes MD, Davey RT Jr; IRC005 Study Team. Beigel JH, et al. Lancet Respir Med. 2019 Nov;7(11):941-950. doi: 10.1016/S2213-2600(19)30199-7. Epub 2019 Sep 30. Lancet Respir Med. 2019. PMID: 31582360 Free PMC article. Clinical Trial. - Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial.
Groome MJ, Koen A, Fix A, Page N, Jose L, Madhi SA, McNeal M, Dally L, Cho I, Power M, Flores J, Cryz S. Groome MJ, et al. Lancet Infect Dis. 2017 Aug;17(8):843-853. doi: 10.1016/S1473-3099(17)30242-6. Epub 2017 May 5. Lancet Infect Dis. 2017. PMID: 28483414 Free PMC article. Clinical Trial.
Cited by
- Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations.
Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, Shrestha BR, Arabi YM, Ng J, Gomersall CD, Nishimura M, Koh Y, Du B; Asian Critical Care Clinical Trials Group. Phua J, et al. Lancet Respir Med. 2020 May;8(5):506-517. doi: 10.1016/S2213-2600(20)30161-2. Epub 2020 Apr 6. Lancet Respir Med. 2020. PMID: 32272080 Free PMC article. Review. - Prodrug nanotherapy demonstrates in vivo anticryptosporidial efficacy in a mouse model of chronic Cryptosporidium infection.
Ranjan AP, Czyzyk DJ, Martinez-Traverso G, Sadiqova A, Valhondo M, Schaefer DA, Spasov KA, Jorgensen WL, Vishwanatha JK, Riggs MW, Castellanos-Gonzalez A, Anderson KS. Ranjan AP, et al. RSC Pharm. 2024 Sep 19;1(5):963-975. doi: 10.1039/d4pm00093e. eCollection 2024 Dec 10. RSC Pharm. 2024. PMID: 39372445 Free PMC article. - Sensitivity to Vaccines, Therapeutic Antibodies, and Viral Entry Inhibitors and Advances To Counter the SARS-CoV-2 Omicron Variant.
Zhou H, Møhlenberg M, Thakor JC, Tuli HS, Wang P, Assaraf YG, Dhama K, Jiang S. Zhou H, et al. Clin Microbiol Rev. 2022 Sep 21;35(3):e0001422. doi: 10.1128/cmr.00014-22. Epub 2022 Jun 6. Clin Microbiol Rev. 2022. PMID: 35862736 Free PMC article. Review. - Characterization of a human monoclonal antibody generated from a B-cell specific for a prefusion-stabilized spike protein of Middle East respiratory syndrome coronavirus.
Choi JH, Woo HM, Lee TY, Lee SY, Shim SM, Park WJ, Yang JS, Kim JA, Yun MR, Kim DW, Kim SS, Zhang Y, Shi W, Wang L, Graham BS, Mascola JR, Wang N, McLellan JS, Lee JY, Lee H. Choi JH, et al. PLoS One. 2020 May 8;15(5):e0232757. doi: 10.1371/journal.pone.0232757. eCollection 2020. PLoS One. 2020. PMID: 32384116 Free PMC article. - The Principles of Antibody Therapy for Infectious Diseases with Relevance for COVID-19.
Casadevall A, Pirofski LA, Joyner MJ. Casadevall A, et al. mBio. 2021 Mar 2;12(2):e03372-20. doi: 10.1128/mBio.03372-20. mBio. 2021. PMID: 33653885 Free PMC article. Review.
References
- WHO Middle East respiratory syndrome coronavirus (MERS-CoV) Sept 27, 2017. http://www.who.int/emergencies/mers-cov/en/ (accessed Nov 27, 2017).
- Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22:521–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z99 AI999999/ImNIH/Intramural NIH HHS/United States
- HHSN272201100022C/AI/NIAID NIH HHS/United States
- ZIA AI000984-10/ImNIH/Intramural NIH HHS/United States
- HHSN261200800001C/CA/NCI NIH HHS/United States
- HHSN272201100022I/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous