Myocardial Regeneration via Progenitor Cell-Derived Exosomes - PubMed (original) (raw)
Review
Myocardial Regeneration via Progenitor Cell-Derived Exosomes
Janita A Maring et al. Stem Cells Int. 2017.
Abstract
In the past 20 years, a variety of cell products has been evaluated in terms of their capacity to treat patients with acute myocardial infarction and chronic heart failure. Despite initial enthusiasm, therapeutic efficacy has overall been disappointing, and clinical application is costly and complex. Recently, a subset of small extracellular vesicles (EVs), commonly referred to as "exosomes," was shown to confer cardioprotective and regenerative signals at a magnitude similar to that of their donor cells. The conceptual advantage is that they may be produced in industrial quantities and stored at the point-of-care for off-the-shelf application, ideally without eliciting a relevant recipient immune response or other adverse effects associated with viable cells. The body of evidence on beneficial exosome-mediated effects in animal models of heart diseases is rapidly growing. However, there is significant heterogeneity in terms of exosome source cells, isolation process, therapeutic dosage, and delivery mode. This review summarizes the current state of research on exosomes as experimental therapy of heart diseases and seeks to identify roadblocks that need to be overcome prior to clinical application.
Figures
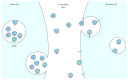
Figure 1
Exosomes are formed by invaginations of intercellular vesicles such as endosomes, which then form multivesicular bodies (MVBs). Exosomes are released into the extracellular space by fusion of the MVB with the cell membrane. Recipient cells take up the exosomes through direct fusion with the cell membrane, through internalisation or through receptor-ligand interaction on the recipient cell membrane.
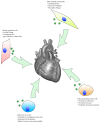
Figure 2
Several cell sources are being examined for use as an exosome therapy, most prominently cardiac progenitor cells, mesenchymal stem cells, and induced pluripotent stem cells as well as embryonic stem cells. Each of them has distinct advantages and disadvantages regarding cardiac regeneration.
Similar articles
- Stem cells-derived exosomes as cardiac regenerative agents.
Farahzadi R, Fathi E, Valipour B, Ghaffary S. Farahzadi R, et al. Int J Cardiol Heart Vasc. 2024 Mar 30;52:101399. doi: 10.1016/j.ijcha.2024.101399. eCollection 2024 Jun. Int J Cardiol Heart Vasc. 2024. PMID: 38584674 Free PMC article. Review. - Therapeutic Potential of Hematopoietic Stem Cell-Derived Exosomes in Cardiovascular Disease.
Radosinska J, Bartekova M. Radosinska J, et al. Adv Exp Med Biol. 2017;998:221-235. doi: 10.1007/978-981-10-4397-0_15. Adv Exp Med Biol. 2017. PMID: 28936743 Review. - Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardiovascular diseases.
Rezaie J, Rahbarghazi R, Pezeshki M, Mazhar M, Yekani F, Khaksar M, Shokrollahi E, Amini H, Hashemzadeh S, Sokullu SE, Tokac M. Rezaie J, et al. J Cell Physiol. 2019 Dec;234(12):21732-21745. doi: 10.1002/jcp.28894. Epub 2019 May 29. J Cell Physiol. 2019. PMID: 31140622 Review. - Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles.
Zhang B, Yeo RW, Tan KH, Lim SK. Zhang B, et al. Int J Mol Sci. 2016 Feb 6;17(2):174. doi: 10.3390/ijms17020174. Int J Mol Sci. 2016. PMID: 26861305 Free PMC article. Review. - Stem cell-derived exosomes - an emerging tool for myocardial regeneration.
Lazar E, Benedek T, Korodi S, Rat N, Lo J, Benedek I. Lazar E, et al. World J Stem Cells. 2018 Aug 26;10(8):106-115. doi: 10.4252/wjsc.v10.i8.106. World J Stem Cells. 2018. PMID: 30190780 Free PMC article. Review.
Cited by
- Extracellular Vesicles in Atherosclerosis: State of the Art.
Olejarz W, Sadowski K, Radoszkiewicz K. Olejarz W, et al. Int J Mol Sci. 2023 Dec 27;25(1):388. doi: 10.3390/ijms25010388. Int J Mol Sci. 2023. PMID: 38203558 Free PMC article. Review. - Extracellular vesicles in nanomedicine and regenerative medicine: A review over the last decade.
Moghassemi S, Dadashzadeh A, Sousa MJ, Vlieghe H, Yang J, León-Félix CM, Amorim CA. Moghassemi S, et al. Bioact Mater. 2024 Mar 2;36:126-156. doi: 10.1016/j.bioactmat.2024.02.021. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38450204 Free PMC article. Review. - Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury.
Liu N, Xie L, Xiao P, Chen X, Kong W, Lou Q, Chen F, Lu X. Liu N, et al. Mol Cell Biochem. 2022 Apr;477(4):1249-1260. doi: 10.1007/s11010-021-04343-7. Epub 2022 Feb 4. Mol Cell Biochem. 2022. PMID: 35119583 Free PMC article. - Cardiomyocytes Cellular Phenotypes After Myocardial Infarction.
Lodrini AM, Goumans MJ. Lodrini AM, et al. Front Cardiovasc Med. 2021 Nov 8;8:750510. doi: 10.3389/fcvm.2021.750510. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34820429 Free PMC article. Review. - Anti-human CD9 antibody Fab fragment impairs the internalization of extracellular vesicles and the nuclear transfer of their cargo proteins.
Santos MF, Rappa G, Karbanová J, Vanier C, Morimoto C, Corbeil D, Lorico A. Santos MF, et al. J Cell Mol Med. 2019 Jun;23(6):4408-4421. doi: 10.1111/jcmm.14334. Epub 2019 Apr 13. J Cell Mol Med. 2019. PMID: 30982221 Free PMC article.
References
- Jeevanantham V., Butler M., Saad A., Abdel-Latif A., Zuba-Surma E. K., Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126(5):551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous