Exceptional Response to Pembrolizumab in a Metastatic, Chemotherapy/Radiation-Resistant Ovarian Cancer Patient Harboring a PD-L1-Genetic Rearrangement - PubMed (original) (raw)
Case Reports
Exceptional Response to Pembrolizumab in a Metastatic, Chemotherapy/Radiation-Resistant Ovarian Cancer Patient Harboring a PD-L1-Genetic Rearrangement
Stefania Bellone et al. Clin Cancer Res. 2018.
Abstract
Purpose: Ovarian carcinoma no longer responsive to surgery and chemotherapy remains an incurable disease. Alternative therapeutic options remain desperately needed.Patients and Methods: We describe a heavily pretreated patient with ovarian cancer with recurrent disease experiencing a remarkable clinical response to treatment with the anti-PD1 immune checkpoint inhibitor pembrolizumab. The clinical, pathological, and genomic characteristics of this exceptional ovarian cancer responder were carefully investigated using immunohistochemistry (IHC), quantitative multiplex fluorescence methods (i.e., automated quantitative analysis, AQUA) and whole-exome sequencing (WES) techniques.Results: The patient harbored a recurrent/metastatic radiation and chemotherapy-resistant high-grade ovarian carcinoma with clear cell features. While progressing on any standard treatment modality, she demonstrated a remarkable complete response to the anti-PD1 immune checkpoint inhibitor pembrolizumab. WES results were notable for the presence a relative low number of mutations (tumor mutation load/Mb = 4.31, total mutations = 164) and a peculiar structural variant disrupting the 3' region of the PD-L1 gene causing aberrant PD-L1 surface expression as confirmed by IHC and AQUA technology. Heavy infiltration of the PD-L1-mutated and PD-L1-overexpressing tumor with T-cell lymphocytes (i.e., CD4+/CD8+ TIL), CD68+ macrophages, and CD20+ B cells was detected in the surgical specimen strongly suggesting immune evasion as a key mechanism of tumor growth and survival. Patient's complete clinical responses remain unchanged at the time of the writing of this report with no significant side effects reported to date.Conclusions: Anti-PD1 inhibitors may represent a novel treatment option for recurrent/metastatic human tumors refractory to salvage treatment harboring PD-L1 gene structural variations causing aberrant PD-L1 expression. Clin Cancer Res; 24(14); 3282-91. ©2018 AACR See related commentary by Lheureux, p. 3233.
©2018 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest: None
Figures
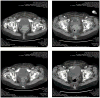
Figure 1
Representative CAT scans demonstrating activity (ie, complete response) to pembrolizumab. Left upper panel: Pretreatment images with baseline measurement of the representative metastatic tumor deposit (ie, pelvic/vaginal mass). Right upper Panel; stability of the lesion after 3 pembrolizumab infusions. Left and Right Lower panels: Complete regression of the metastatic tumor deposits after 16 and 24 weeks from treatment initiation with pembrolizumab.
Figure 2
Whole exome sequencing results. (A) Quality assessment and quality control of sequencing data for tumor and matched normal samples. (B) Somatic mutation classification. (C) The distribution of six different substitution subtypes. (D) Potential neoantigen classification. Strong: IC50 ≤ 50 nM, Intermediate: 50 nM < IC50 ≤ 150 nM, Weak: 150 nM < IC50 ≤ 500 nM. (E) Integrative Genomics Viewer (IGV) plots showing genomic rearrangement involving the PD-L1 gene. The event consists in a translocation/insertion of a 5′ 32 nucleotide fragment from the exon 5 of the PLGRKT gene, in the 5′ end of the PD-L1 gene, resulting in a breakpoint in exon 7 (3′ UTR). Green reads indicate the tandem duplication. Grey reads display normal sequencing reads.
Figure 3
Whole exome sequencing results. Somatic mutations (SNV), in cancer or immune-related genes and copy number variations (CNV) in cancer and immune related genes.
Figure 4
High grade ovarian carcinoma with marked peritumoral inflammatory cell infiltrate (A). The tumor cells and peritumoral inflammatory cells show moderately to intense membranous staining with PD-L1 immunohistochemistry (B). T-lymphocytes represent the predominant component among the immune cells, highlighted by CD4 (C) and CD8 (D) immunostains. B-lymphocytes (E: CD20 immunostain) and macrophages (F: CD68 immunostain) are present in a smaller proportion. CD56 and TIA immunostains were negative for NK-cells (image not shown). (A: hematoxylin-eosin stain, B: PD-L1 immunostain, C: CD4 immunostain, D: CD8 immunostain, E: CD20 immunostain, F: CD68 immunostain; all images at 200x original magnification).
Figure 5
Detection of PD-L1 protein expression in tumor cells using immunofluorescence multiplexing panel. A–F, Multiplex IF panel of PD-L1 (red)/Cytokeratin (green)/DAPI (blue)/CD68 (magenta) of the same region. A–D, Representative images of single channels (DAPI, CK, PD-L1, CD68) of the multiplex IF panel. E, Representative fluorescence image showing the colocalization of PD-L1 and cytokeratin. CD68+ macrophages do not express PD-L1. F, H&E staining. G–L, Multiplex IF panel of PD-L1 (red)/cytokeratin (green)/DAPI (blue)/CD8 (magenta) of the same region. G–J, Representative images of single channels (DAPI, CK, PD-L1, CD8) of the multiplex IF panel. K, Representative fluorescence image showing the colocalization of PD-L1 (red) and cytokeratin (green). CD8 (magenta)+ T cells do not express PD-L1. L, H&E staining.
Figure 6
PD-L1 expression by immunohistochemistry in representative high grade ovarian serous carcinomas from the TMA. A: Focal PD-L1 expression (between 1–50%) in tumor cells. C, D, E: No PD-L1 expression is identified in tumor cells. (All images at 200x original magnification).
Comment in
- Biomarker Discovery from We to Me: Is Learning from Each Patient a New Approach?
Lheureux S. Lheureux S. Clin Cancer Res. 2018 Jul 15;24(14):3233-3235. doi: 10.1158/1078-0432.CCR-18-0380. Epub 2018 Mar 26. Clin Cancer Res. 2018. PMID: 29581132
Similar articles
- Real-world outcomes of anti-PD1 antibodies in platinum-refractory, PD-L1-positive recurrent and/or metastatic non-small cell lung cancer, and its potential practical predictors: first report from Korean Cancer Study Group LU19-05.
Park JH, You GL, Ahn MJ, Kim SW, Hong MH, Han JY, Ock CY, Lee JS, Oh IJ, Lee SY, Kim CH, Min YJ, Choi YH, Ryu JS, Park SH, Ahn HK, Shim BY, Lee KH, Lee SY, Kim JS, Yi J, Choi SK, An H, Kang JH. Park JH, et al. J Cancer Res Clin Oncol. 2021 Aug;147(8):2459-2469. doi: 10.1007/s00432-021-03527-4. Epub 2021 Feb 1. J Cancer Res Clin Oncol. 2021. PMID: 33523301 - The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis.
Zhang T, Xie J, Arai S, Wang L, Shi X, Shi N, Ma F, Chen S, Huang L, Yang L, Ma W, Zhang B, Han W, Xia J, Chen H, Zhang Y. Zhang T, et al. Oncotarget. 2016 Nov 8;7(45):73068-73079. doi: 10.18632/oncotarget.12230. Oncotarget. 2016. PMID: 27683031 Free PMC article. - [Efficacy of PD-1/PD-L1 immune checkpoint inhibitors and PD-L1 testing in thoracic cancers].
Duruisseaux M, Rouquette I, Adam J, Cortot A, Cazes A, Gibault L, Damotte D, Lantuejoul S. Duruisseaux M, et al. Ann Pathol. 2017 Feb;37(1):61-78. doi: 10.1016/j.annpat.2016.12.009. Epub 2017 Feb 3. Ann Pathol. 2017. PMID: 28162296 Review. French. - T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028.
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK. Ott PA, et al. J Clin Oncol. 2019 Feb 1;37(4):318-327. doi: 10.1200/JCO.2018.78.2276. Epub 2018 Dec 13. J Clin Oncol. 2019. PMID: 30557521 Clinical Trial. - Combination therapy with PD-1 or PD-L1 inhibitors for cancer.
Hayashi H, Nakagawa K. Hayashi H, et al. Int J Clin Oncol. 2020 May;25(5):818-830. doi: 10.1007/s10147-019-01548-1. Epub 2019 Sep 23. Int J Clin Oncol. 2020. PMID: 31549270 Review.
Cited by
- Pan-cancer Landscape of Programmed Death Ligand-1 and Programmed Death Ligand-2 Structural Variations.
Hoskins EL, Samorodnitsky E, Wing MR, Reeser JW, Hopkins JF, Murugesan K, Kuang Z, Vella R, Stein L, Risch Z, Yu L, Adebola S, Paruchuri A, Carpten J, Chahoud J, Edge S, Kolesar J, McCarter M, Nepple KG, Reilley M, Scaife C, Tripathi A, Single N, Huang RSP, Albacker LA, Roychowdhury S. Hoskins EL, et al. JCO Precis Oncol. 2023 Jan;7:e2200300. doi: 10.1200/PO.22.00300. JCO Precis Oncol. 2023. PMID: 36623238 Free PMC article. - Quantitative Assessment and Prognostic Associations of the Immune Landscape in Ovarian Clear Cell Carcinoma.
Khalique S, Nash S, Mansfield D, Wampfler J, Attygale A, Vroobel K, Kemp H, Buus R, Cottom H, Roxanis I, Jones T, von Loga K, Begum D, Guppy N, Ramagiri P, Fenwick K, Matthews N, Hubank MJF, Lord CJ, Haider S, Melcher A, Banerjee S, Natrajan R. Khalique S, et al. Cancers (Basel). 2021 Jul 30;13(15):3854. doi: 10.3390/cancers13153854. Cancers (Basel). 2021. PMID: 34359755 Free PMC article. - The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy.
Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. Rodriguez GM, et al. Cancers (Basel). 2018 Jul 24;10(8):242. doi: 10.3390/cancers10080242. Cancers (Basel). 2018. PMID: 30042343 Free PMC article. Review. - Pathogenesis and biomarkers of natural killer T cell lymphoma (NKTL).
Somasundaram N, Lim JQ, Ong CK, Lim ST. Somasundaram N, et al. J Hematol Oncol. 2019 Mar 15;12(1):28. doi: 10.1186/s13045-019-0717-6. J Hematol Oncol. 2019. PMID: 30876435 Free PMC article. Review. - Personalized Versus Precision Nanomedicine for Treatment of Ovarian Cancer.
Garbuzenko OB, Sapiezynski J, Girda E, Rodriguez-Rodriguez L, Minko T. Garbuzenko OB, et al. Small. 2024 Oct;20(41):e2307462. doi: 10.1002/smll.202307462. Epub 2024 Feb 11. Small. 2024. PMID: 38342698
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. - PubMed
- Creasman DiSaia, DiSaia PJ, Creasman WT., editors. Clinical Gynecologic Oncology. 8. Saunders Elsevier Inc; Philadelphia, PA: 2012.
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous