Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan - PubMed (original) (raw)
Review
Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan
Tuan Leng Tay et al. Front Mol Neurosci. 2018.
Abstract
Microglia are the predominant immune response cells and professional phagocytes of the central nervous system (CNS) that have been shown to be important for brain development and homeostasis. These cells present a broad spectrum of phenotypes across stages of the lifespan and especially in CNS diseases. Their prevalence in all neurological pathologies makes it pertinent to reexamine their distinct roles during steady-state and disease conditions. A major question in the field is determining whether the clustering and phenotypical transformation of microglial cells are leading causes of pathogenesis, or potentially neuroprotective responses to the onset of disease. The recent explosive growth in our understanding of the origin and homeostasis of microglia, uncovering their roles in shaping of the neural circuitry and synaptic plasticity, allows us to discuss their emerging functions in the contexts of cognitive control and psychiatric disorders. The distinct mesodermal origin and genetic signature of microglia in contrast to other neuroglial cells also make them an interesting target for the development of therapeutics. Here, we review the physiological roles of microglia, their contribution to the effects of environmental risk factors (e.g., maternal infection, early-life stress, dietary imbalance), and their impact on psychiatric disorders initiated during development (e.g., Nasu-Hakola disease (NHD), hereditary diffuse leukoencephaly with spheroids, Rett syndrome, autism spectrum disorders (ASDs), and obsessive-compulsive disorder (OCD)) or adulthood (e.g., alcohol and drug abuse, major depressive disorder (MDD), bipolar disorder (BD), schizophrenia, eating disorders and sleep disorders). Furthermore, we discuss the changes in microglial functions in the context of cognitive aging, and review their implication in neurodegenerative diseases of the aged adult (e.g., Alzheimer's and Parkinson's). Taking into account the recent identification of microglia-specific markers, and the availability of compounds that target these cells selectively in vivo, we consider the prospect of disease intervention via the microglial route.
Keywords: aging; autism spectrum disorder; early-life stress; major depressive disorder; microglia; microgliopathies; neurodegenerative disease; schizophrenia.
Figures
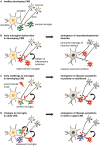
Figure 1
Models of non-physiological microglia and their impacts on the onset of disease. (A) Normal microglia-neuron interactions in the central nervous system (CNS). (B) An early microglial dysfunction due to genetic or environmental (e.g., maternal or perinatal stress, inflammation, dietary deficiency) risk factors can lead to impaired neuronal functions and an early emergence of neurodevelopmental disorders. Aberrant release of cytokines, impaired pruning and phagocytic activities can affect neuronal densities, maturation and wiring, thus translating into permanent defects of the neural network. These include an imbalance of excitability to inhibition, or altered connectivity between brain regions, which are sufficient to induce the onset of psychiatric disorders during childhood (e.g., Nasu-Hakola disease (NHD), hereditary diffuse leukoencephaly with spheroids (HDLS), Rett syndrome (RTT), autism spectrum disorder (ASD) and obsessive-compulsive disorder (OCD)), or render an individual vulnerable to subsequent insults. (C) An early environmental challenge can prime microglia by altering their maturation and inflammatory states with limited immediate impacts on the neuronal network, thus resulting in asymptomatic changes. However, primed microglia are rendered more susceptible to subsequent challenges such as stress or chronic infections, and may adopt abnormal patterns of cytokine secretion or synaptic pruning later in life. These changes may progressively damage the neural system during puberty and adulthood, leading to the emergence of psychiatric disorders (e.g., alcohol and drug abuse, major depressive disorder (MDD), schizophrenia, bipolar disorder (BD), eating disorders and sleep disorders). (D) Changes to microglial phenotypes occurring during adulthood may be accelerated by genetic or environmental factors. Non-physiological microglia may have reduced capability to restore CNS homeostasis, or contribute to neurodegeneration and altered wiring, which result in the onset of cognitive disorders in adult [as in **(C)**] and aged patients (e.g., Alzheimer’s disease (AD), dementia and Parkinson’s disease (PD)).
Figure 2
Genetic and environmental implications for the role of microglia in the progression of psychiatric diseases across the lifespan. Pathologies caused by, or linked to, genetic risk factors are marked with an orange star. Disease-associated priming of microglia due to environmental risk factors, such as perinatal inflammation, maternal and early life stress, dietary imbalance and chronic stress during adulthood, is indicated with a gray star.
Figure 3
Typical vs. dark microglia. (A) Typical microglia (m) that displays a light cytoplasm (ct) and nucleoplasm (np) with a clearly defined heterochromatin (hc) pattern, as well as intact organelles in the hypothalamus of a healthy adult mouse. Mitochondrion = mt; ma = myelinated axon. (B) Dark microglia (dm) in the hippocampus of a chronically-stressed Cx3cr1 knockout mouse showing various signs of oxidative stress, including the darkening/condensation of its cytoplasm and nucleoplasm, making it appear as dark as mitochondria, and dilation of its endoplasmic reticulum (er) bv, blood vessel. (C) Processes from typical microglia are generally bulky and make focal contacts with synaptic elements. Several phagocytic inclusions (in) are shown in an IBA1-stained process, in addition to a synapse between an axon terminal (t) and a dendritic spine (s; colored in purple), within the hippocampus of a healthy adult mouse. (D) By contrast, dark microglia’s processes extensively encircle synaptic elements (colored in purple), including shrunken terminals undergoing digestion, which are surrounded by extended extracellular space (asterisk), and entire excitatory synapses, as shown in the hippocampus of a chronically-stressed Cx3cr1 knockout mouse. Microglial contacts with synaptic clefts are indicated by arrowheads in (C,D). Blood vessels, cells and cellular elements are labeled by the large bold font. Organelles and subcellular compartments are labeled by the smaller font.
Figure 4
Effects of microglial targeting in animal models and human patients discussed in this review. (A) Temporal effects of transient microglial depletion in rodents and nonhuman primates across the lifespan. (B) Potential clinical effects of minocycline on modulating the roles of microglia in adult psychiatric diseases.
Similar articles
- Microglia and Brain Disorders: The Role of Vitamin D and Its Receptor.
Mirarchi A, Albi E, Beccari T, Arcuri C. Mirarchi A, et al. Int J Mol Sci. 2023 Jul 25;24(15):11892. doi: 10.3390/ijms241511892. Int J Mol Sci. 2023. PMID: 37569267 Free PMC article. Review. - The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS.
Arcuri C, Mecca C, Bianchi R, Giambanco I, Donato R. Arcuri C, et al. Front Mol Neurosci. 2017 Jun 19;10:191. doi: 10.3389/fnmol.2017.00191. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28674485 Free PMC article. Review. - Microglia across the lifespan: from origin to function in brain development, plasticity and cognition.
Tay TL, Savage JC, Hui CW, Bisht K, Tremblay MÈ. Tay TL, et al. J Physiol. 2017 Mar 15;595(6):1929-1945. doi: 10.1113/JP272134. Epub 2016 May 29. J Physiol. 2017. PMID: 27104646 Free PMC article. Review. - Primary Microglia Dysfunction or Microgliopathy: A Cause of Dementias and Other Neurological or Psychiatric Disorders.
Bianchin MM, Snow Z. Bianchin MM, et al. Neuroscience. 2022 Aug 10;497:324-339. doi: 10.1016/j.neuroscience.2022.06.032. Epub 2022 Jun 24. Neuroscience. 2022. PMID: 35760218 Review. - Microglial functional alteration and increased diversity in the challenged brain: Insights into novel targets for intervention.
Tremblay MÈ. Tremblay MÈ. Brain Behav Immun Health. 2021 Jul 20;16:100301. doi: 10.1016/j.bbih.2021.100301. eCollection 2021 Oct. Brain Behav Immun Health. 2021. PMID: 34589793 Free PMC article.
Cited by
- Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet.
Bordeleau M, Lacabanne C, Fernández de Cossío L, Vernoux N, Savage JC, González-Ibáñez F, Tremblay MÈ. Bordeleau M, et al. J Neuroinflammation. 2020 Sep 5;17(1):264. doi: 10.1186/s12974-020-01914-1. J Neuroinflammation. 2020. PMID: 32891154 Free PMC article. - Early Adversity and Accelerated Brain Aging: A Mini-Review.
Chaudhari PR, Singla A, Vaidya VA. Chaudhari PR, et al. Front Mol Neurosci. 2022 Mar 22;15:822917. doi: 10.3389/fnmol.2022.822917. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35392273 Free PMC article. Review. - An Atlas of Genetic Correlations and Genetically Informed Associations Linking Psychiatric and Immune-Related Phenotypes.
Tylee DS, Lee YK, Wendt FR, Pathak GA, Levey DF, De Angelis F, Gelernter J, Polimanti R. Tylee DS, et al. JAMA Psychiatry. 2022 Jul 1;79(7):667-676. doi: 10.1001/jamapsychiatry.2022.0914. JAMA Psychiatry. 2022. PMID: 35507366 Free PMC article. - Contribution of Age, Brain Region, Mood Disorder Pathology, and Interindividual Factors on the Methylome of Human Microglia.
de Witte LD, Wang Z, Snijders GLJL, Mendelev N, Liu Q, Sneeboer MAM, Boks MPM, Ge Y, Haghighi F. de Witte LD, et al. Biol Psychiatry. 2022 Mar 15;91(6):572-581. doi: 10.1016/j.biopsych.2021.10.020. Epub 2021 Oct 30. Biol Psychiatry. 2022. PMID: 35027166 Free PMC article. - Alterations in the Nervous System and Gut Microbiota after _β_-Hemolytic Streptococcus Group A Infection-Characteristics and Diagnostic Criteria of PANDAS Recognition.
Baj J, Sitarz E, Forma A, Wróblewska K, Karakuła-Juchnowicz H. Baj J, et al. Int J Mol Sci. 2020 Feb 21;21(4):1476. doi: 10.3390/ijms21041476. Int J Mol Sci. 2020. PMID: 32098238 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources