Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics - PubMed (original) (raw)
Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics
Brandon L Scott et al. Nat Commun. 2018.
Abstract
Clathrin-mediated endocytosis (CME) internalizes plasma membrane by reshaping small regions of the cell surface into spherical vesicles. The key mechanistic question of how coat assembly produces membrane curvature has been studied with molecular and cellular structural biology approaches, without direct visualization of the process in living cells; resulting in two competing models for membrane bending. Here we use polarized total internal reflection fluorescence microscopy (pol-TIRF) combined with electron, atomic force, and super-resolution optical microscopy to measure membrane curvature during CME. Surprisingly, coat assembly accommodates membrane bending concurrent with or after the assembly of the clathrin lattice. Once curvature began, CME proceeded to scission with robust timing. Four color pol-TIRF showed that CALM accumulated at high levels during membrane bending, implicating its auxiliary role in curvature generation. We conclude that clathrin-coat assembly is versatile and that multiple membrane-bending trajectories likely reflect the energetics of coat assembly relative to competing forces.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
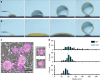
Mechanisms of membrane bending during CME and clathrin ultrastructure in unroofed SK-MEL-2 cells. a Schematic representation of CME in which membrane bending proceeds with a fixed radius of curvature during the addition of clathrin subunits. b Representation of CME in which clathrin first assembles into a flat sheet that remodels into a vesicle. c Correlative dSTORM and platinum-replica TEM images of fluorescently labeled clathrin (magenta) demonstrate a range of heterogeneous topographies even at the earliest stages of CME; scale bar is 200 nm. d The size distribution observed amongst clathrin structures in WT SK-MEL-2 cells (black) or SK-MEL-2 cells exogenously expressing clathrin light chain for correlative microscopy (blue). The inset shows ratios of average fluorescence associated with round vs. domed structures and domed vs. flat structures (SD shown for N = 3 cell membranes)
Fig. 2
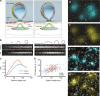
Polarized-TIRF microscopy enables imaging of membrane bending at clathrin-coated structures. a Schematic representation of pol-TIRF. DiI–C18 orients its dipole moment with the plasma membrane. S-polarized TIRF illuminates horizontal dye molecules, whereas P-polarized TIRF selectively excites vertical dye molecules. The P/S provides contrast for membrane bending. b Simulation of pol-TIRF signals for Class 1 and Class 2 membrane bending. c Quantification of high-resolution simulation at 10 discrete points for Class 1 (blue) and Class 2 (orange) in the absence of noise. d Correlative TEM-pol-TIRF imaging. Overlay of fluorescence from endogenous clathrin-Tq2 on the TEM micrograph showing four clathrin structures; scale bar is 100 nm. e Overlay of P/S on the same region of the micrograph. f Correlative AFM-pol-TIRF imaging; Overlay of clathrin-Tq2 on AFM micrograph; scale bar is 250 nm. g Overlay of P/S signal on the same region of the AFM micrograph. h Quantification of P/S intensity and heights from correlative tomographic reconstructions (blue), and AFM (orange)
Fig. 3
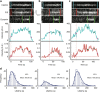
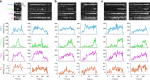
Distinct modes of membrane bending observed by pol-TIRF. a–c Kymographs of clathrin-Tq2, membrane bending (P/S), dynamin-eGFP, and corresponding intensity traces for clathrin (cyan) and P/S (red) for the three classes of membrane bending observed by pol-TIRF. The colored lines indicate the lifetime for each event, the dashed red line highlights the start of P/S event. a Class 1: clathrin and P/S signals proceed together indicating membrane bending as clathrin assembles. b Class 2: clathrin signal plateaus prior to the start of P/S indicating all required clathrin was present as a flat sheet. c Class 3: clathrin assembles prior to P/S signal, but new clathrin was recruited as the membrane bends and the vesicle is formed. d The clathrin lifetime histogram of each class from an average of four cells (N = 481 tracks total)
Fig. 4
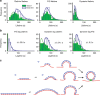
Lifetime analysis of clathrin events. a Lifetime distribution for clathrin, P/S, and dynamin; Class 1, green bars and line, Class 2/3 open with blue line. b Lag time for P/S and dynamin relative to the start of the clathrin events, and lag time for the start of dynamin relative to the start of P/S. c Relationship between clathrin assembly and membrane bending during CME
Fig. 5
CALM recruitment is highly correlated with membrane bending. a pol-TIRF kymographs and intensity traces of clathrin, dynamin, CALM, and P/S are shown for Class 1 (a), Class 2 (b), and Class 3 (c)
Similar articles
- ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat.
Avinoam O, Schorb M, Beese CJ, Briggs JA, Kaksonen M. Avinoam O, et al. Science. 2015 Jun 19;348(6241):1369-72. doi: 10.1126/science.aaa9555. Science. 2015. PMID: 26089517 - The AP2 adaptor enhances clathrin coat stiffness.
Lherbette M, Redlingshöfer L, Brodsky FM, Schaap IAT, Dannhauser PN. Lherbette M, et al. FEBS J. 2019 Oct;286(20):4074-4085. doi: 10.1111/febs.14961. Epub 2019 Jul 3. FEBS J. 2019. PMID: 31199077 Free PMC article. - Induced nanoscale membrane curvature bypasses the essential endocytic function of clathrin.
Cail RC, Shirazinejad CR, Drubin DG. Cail RC, et al. J Cell Biol. 2022 Jul 4;221(7):e202109013. doi: 10.1083/jcb.202109013. Epub 2022 May 9. J Cell Biol. 2022. PMID: 35532382 Free PMC article. - Mechanistic divergences of endocytic clathrin-coated vesicle formation in mammals, yeasts and plants.
Johnson A. Johnson A. J Cell Sci. 2024 Aug 15;137(16):jcs261847. doi: 10.1242/jcs.261847. Epub 2024 Aug 20. J Cell Sci. 2024. PMID: 39161994 Free PMC article. Review. - Mechanisms of clathrin-mediated endocytosis.
Kaksonen M, Roux A. Kaksonen M, et al. Nat Rev Mol Cell Biol. 2018 May;19(5):313-326. doi: 10.1038/nrm.2017.132. Epub 2018 Feb 7. Nat Rev Mol Cell Biol. 2018. PMID: 29410531 Review.
Cited by
- Evolutionarily unique mechanistic framework of clathrin-mediated endocytosis in plants.
Narasimhan M, Johnson A, Prizak R, Kaufmann WA, Tan S, Casillas-Pérez B, Friml J. Narasimhan M, et al. Elife. 2020 Jan 23;9:e52067. doi: 10.7554/eLife.52067. Elife. 2020. PMID: 31971511 Free PMC article. - Structure of the endocytic adaptor complex reveals the basis for efficient membrane anchoring during clathrin-mediated endocytosis.
Lizarrondo J, Klebl DP, Niebling S, Abella M, Schroer MA, Mertens HDT, Veith K, Thuenauer R, Svergun DI, Skruzny M, Sobott F, Muench SP, Garcia-Alai MM. Lizarrondo J, et al. Nat Commun. 2021 May 17;12(1):2889. doi: 10.1038/s41467-021-23151-7. Nat Commun. 2021. PMID: 34001871 Free PMC article. - Engineered IgG1-Fc Molecules Define Valency Control of Cell Surface Fcγ Receptor Inhibition and Activation in Endosomes.
Bailey EM, Choudhury A, Vuppula H, Ortiz DF, Schaeck J, Manning AM, Bosques CJ, Hoppe AD. Bailey EM, et al. Front Immunol. 2021 Feb 15;11:617767. doi: 10.3389/fimmu.2020.617767. eCollection 2020. Front Immunol. 2021. PMID: 33679705 Free PMC article. - Unravelling the Mystery inside Cells by Using Single-Molecule Fluorescence Imaging.
Zalejski J, Sun J, Sharma A. Zalejski J, et al. J Imaging. 2023 Sep 19;9(9):192. doi: 10.3390/jimaging9090192. J Imaging. 2023. PMID: 37754956 Free PMC article. Review. - Nanodissected elastically loaded clathrin lattices relax to increased curvature.
Tagiltsev G, Haselwandter CA, Scheuring S. Tagiltsev G, et al. Sci Adv. 2021 Aug 13;7(33):eabg9934. doi: 10.1126/sciadv.abg9934. Print 2021 Aug. Sci Adv. 2021. PMID: 34389539 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials