Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway - PubMed (original) (raw)
Resistant starch prevents tumorigenesis of dimethylhydrazine-induced colon tumors via regulation of an ER stress-mediated mitochondrial apoptosis pathway
Qiuyu Wang et al. Int J Mol Med. 2018 Apr.
Abstract
Resistant starch is as common soluble fiber that escapes digestion in the small intestine and can regulate intestinal function, metabolism of blood glucose and lipids, and may prevent tumorigenesis of gastrointestinal cancer. Epidemiology and other evidence have suggested that resistant starch may prevent colon cancer development. The aim of the current study was to explore the ameliorative effects and potential mechanisms of resistant starch in the tumorigenesis of colon tumors induced by dimethylhydrazine in C57BL/6 mice. Western blot analysis, ELISA, microscopy, immunofluorescence and immunohistochemistry were used to analyze the efficacy of resistant starch on the metabolic balance in the colon and tumorigenesis of colon tumors. The results demonstrated that a diet containing resistant starch decreased the animal body weight and reduced free ammonia, pH and short chain fatty acids in feces compared with mice that received a standard diet. Resistant starch reduced the incidence of colon tumors and suppressed the expression of carcinogenesis‑associated proteins, including heat shock protein 25, protein kinase C‑d and gastrointestinal glutathione peroxidase in colon epithelial cells compared with standard starch and control groups. Colon tumor cells proliferation and dedifferentiation were significantly decreased by a resistant starch diet. The results also demonstrated that resistant starch increased the apoptosis of colon tumor cells through regulation of apoptosis‑associated gene expression levels in colon tumor cells. Oxidative stress and endoplasmic reticulum stress were upregulated, and elevation eukaryotic translation initiation factor 2α (eIF2α), activating transcription factor‑4 and secretase‑β expression levels were increased in the resistant starch diet group. Additionally, the activity of eIF2α and PERK were increased in colon tumor cells from mice that had received resistant starch. Increasing DNA damage‑inducible transcript 3 protein (CHOP), binding immunoglobulin protein (BIP) and caspase‑12 expression levels upregulated by resistant starch diet may contribute to the resistant starch‑induced apoptosis of colon tumor cells induced by 1,2‑dimethylhydrazine. In vitro assays demonstrated that knockdown of eIF2α inhibited apoptosis of colon tumor cells isolated from mice fed with resistant starch, which also downregulated CHOP, BIP and caspase‑3 expression levels compared with controls. Furthermore, long‑term survival of experimental mice was prolonged by the resistant starch diet compared with the standard diet group. In conclusion, the results indicate that resistant starch in the diet may prevent carcinogenesis of colon epithelial cells, mediated by enhancing apoptosis through an endoplasmic reticulum stress‑mediated mitochondrial apoptosis pathway.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures
Figure 1
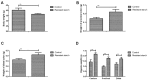
Effects of resistant starch on body weight and metabolic characteristic of colon tissues. (A) Body weight of experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Resistant starch improves weight of (B) proximal colon and (C) distal colon compared to regular diets. (D) Effects of resistant starch on digesta weight in caecum, proximal colon and distal colon in experimental mice induced by 1,2-dimethylhydrazine. (E) Digesta pH in caecum, proximal colon and distal colon in experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Effects of resistant starch on (F) free ammonia, (G) pH and (H) SCFA in faeces in mice fed with resistant starch. *P<0.05 and **P<0.01. SCFA, short chain fatty acids.
Figure 1
Effects of resistant starch on body weight and metabolic characteristic of colon tissues. (A) Body weight of experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Resistant starch improves weight of (B) proximal colon and (C) distal colon compared to regular diets. (D) Effects of resistant starch on digesta weight in caecum, proximal colon and distal colon in experimental mice induced by 1,2-dimethylhydrazine. (E) Digesta pH in caecum, proximal colon and distal colon in experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Effects of resistant starch on (F) free ammonia, (G) pH and (H) SCFA in faeces in mice fed with resistant starch. *P<0.05 and **P<0.01. SCFA, short chain fatty acids.
Figure 2
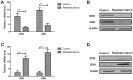
Effects of resistant starch on tumorigenesis in colon tissues induced by 1,2-dimethylhydrazine. (A) Resistant starch inhibits tumor formation in experimental mice treated by 1,2-dimethylhydrazine. (B) Effects of resistant starch decreases incidence of colon tumor in a mouse model induced by 1,2-dimethylhydrazine. (C) Gene and (D) protein expression levels of HSP25, PKC-d and GI-GPx in colon epithelial cells in experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Effects of resistant starch on (E) gene and (F) protein expression levels of c-myc, Ras and p53 in colon epithelial cells. (G) Resistant starch diet inhibits formation of microtubule and tumor vessel in colon tissues in experimental mice. (H) Resistant starch diet inhibits protein express levels of MAT-1 and NRP-2 in colon tissues in experimental mice. **P<0.01. HSP25, heat shock protein 25; PKC-d, protein kinase C-d; GI-GPx, gastrointestinal glutathione peroxidase; MAT-1, CDK-activating kinase assembly factor MAT1; NRP-2, neuropilin-2 (magnification, ×40).
Figure 2
Effects of resistant starch on tumorigenesis in colon tissues induced by 1,2-dimethylhydrazine. (A) Resistant starch inhibits tumor formation in experimental mice treated by 1,2-dimethylhydrazine. (B) Effects of resistant starch decreases incidence of colon tumor in a mouse model induced by 1,2-dimethylhydrazine. (C) Gene and (D) protein expression levels of HSP25, PKC-d and GI-GPx in colon epithelial cells in experimental mice fed with resistant starch after induced by 1,2-dimethylhydrazine. Effects of resistant starch on (E) gene and (F) protein expression levels of c-myc, Ras and p53 in colon epithelial cells. (G) Resistant starch diet inhibits formation of microtubule and tumor vessel in colon tissues in experimental mice. (H) Resistant starch diet inhibits protein express levels of MAT-1 and NRP-2 in colon tissues in experimental mice. **P<0.01. HSP25, heat shock protein 25; PKC-d, protein kinase C-d; GI-GPx, gastrointestinal glutathione peroxidase; MAT-1, CDK-activating kinase assembly factor MAT1; NRP-2, neuropilin-2 (magnification, ×40).
Figure 3
Effects of resistant starch on inhibition of colon tumor cells proliferation and differentiation. (A) Resistant starch diet inhibits colon tumor cells proliferation in colon tissues induced by 1,2-dimethylhydrazine. Effects of resistant starch on (B) gene and (C) protein expression levels of PCNA, claudin 1 and claudin 2 in colon tumor cells. (D) Resistant starch diet inhibits colon tumor cells differentiation in colon tissues induced by 1,2-dimethylhydrazine. Resistant starch diet blocks (E) gene and (F) protein expression levels of mTOR and HK-II in colon tumor cells. (G) Resistant starch diet arrests S phase of colon tumor cells in experimental mice. (H) Long-term survival rate of experimental mice between resistant starch diet and standard diet groups determined by Kaplan-Mrier. **P<0.01. PCNA, proliferating cell nuclear antigen; mTOR, mechanistic target of rapamycin kinase; HK-II, hexokinase-2 (magnification, ×40).
Figure 3
Effects of resistant starch on inhibition of colon tumor cells proliferation and differentiation. (A) Resistant starch diet inhibits colon tumor cells proliferation in colon tissues induced by 1,2-dimethylhydrazine. Effects of resistant starch on (B) gene and (C) protein expression levels of PCNA, claudin 1 and claudin 2 in colon tumor cells. (D) Resistant starch diet inhibits colon tumor cells differentiation in colon tissues induced by 1,2-dimethylhydrazine. Resistant starch diet blocks (E) gene and (F) protein expression levels of mTOR and HK-II in colon tumor cells. (G) Resistant starch diet arrests S phase of colon tumor cells in experimental mice. (H) Long-term survival rate of experimental mice between resistant starch diet and standard diet groups determined by Kaplan-Mrier. **P<0.01. PCNA, proliferating cell nuclear antigen; mTOR, mechanistic target of rapamycin kinase; HK-II, hexokinase-2 (magnification, ×40).
Figure 4
Resistant starch diet induces apoptosis of colon tumors through mitochondrial apoptotic pathway. (A) Resistant starch diet promotes apoptosis of colon tumor cells in mice treated by 1,2-dimethylhydrazine. (B) Effects of resistant starch diet on mitochondria damage in colon tumor cells in experimental mice (magnification, ×100). Resistant starch diet increases pro-apoptosis (C) gene and (D) protein expression levels of cleaved caspase-3 and caspase-9 in colon tumor cells induced by 1,2-dimethylhydrazine. Resistant starch diet increases anti-apoptosis (E) gene and (F) protein expression levels of p53 and Bcl-2 in colon tumor cells induced by 1,2-dimethylhydrazine. Resistant starch diet promotes expression levels of Apaf-1 and Bad in colon tumor tissue in mice treated by 1,2-dimethylhydrazine determined by (G) immunohistochemistry (tissue) and (H) immunofluorescence (cells) (magnification, ×40). **P<0.01. Bcl-2, apoptosis regulator Bcl-2; Apaf-1, apoptotic protease-activating factor 1; Bad, Bcl-2-associated agonist of cell death.
Figure 4
Resistant starch diet induces apoptosis of colon tumors through mitochondrial apoptotic pathway. (A) Resistant starch diet promotes apoptosis of colon tumor cells in mice treated by 1,2-dimethylhydrazine. (B) Effects of resistant starch diet on mitochondria damage in colon tumor cells in experimental mice (magnification, ×100). Resistant starch diet increases pro-apoptosis (C) gene and (D) protein expression levels of cleaved caspase-3 and caspase-9 in colon tumor cells induced by 1,2-dimethylhydrazine. Resistant starch diet increases anti-apoptosis (E) gene and (F) protein expression levels of p53 and Bcl-2 in colon tumor cells induced by 1,2-dimethylhydrazine. Resistant starch diet promotes expression levels of Apaf-1 and Bad in colon tumor tissue in mice treated by 1,2-dimethylhydrazine determined by (G) immunohistochemistry (tissue) and (H) immunofluorescence (cells) (magnification, ×40). **P<0.01. Bcl-2, apoptosis regulator Bcl-2; Apaf-1, apoptotic protease-activating factor 1; Bad, Bcl-2-associated agonist of cell death.
Figure 5
Resistant starch diet increases oxidative stress in colon tumors through regulation of autophagy progression. Effects of resistant starch on (A) mRNA and (B) protein levels of SOD and GSH in colon tumor cells. Effects of resistant starch on (C) mRNA and (D) protein levels of SOD and GSH in colon epithelial cells. (E) Resistant starch upregulates AMPK activity in colon tumor cells. (F) Resistant starch upregulates expression levels of DDIT3 and Beclin 1 in colon tumor cells in mice treated by 1,2-dimethylhydrazine. Resistant starch diet promotes (G) mitophagy and (H) reticulophagy of colon tumor cells in mice treated by 1,2-dimethylhydrazine (magnification, ×40). **P<0.01. SOD, superoxide dismutase; GSH, glutathione synthetase; AMPK, AMP-activated protein kinase; DDIT3, DNA damage-inducible transcript 3 protein; MAP1LC3A, microtubule associated protein 1 light chain 3 α; TOMM20, translocase of outer mitochondrial membrane 20; Neo (Nase), 5′-Nase-ALPase.
Figure 5
Resistant starch diet increases oxidative stress in colon tumors through regulation of autophagy progression. Effects of resistant starch on (A) mRNA and (B) protein levels of SOD and GSH in colon tumor cells. Effects of resistant starch on (C) mRNA and (D) protein levels of SOD and GSH in colon epithelial cells. (E) Resistant starch upregulates AMPK activity in colon tumor cells. (F) Resistant starch upregulates expression levels of DDIT3 and Beclin 1 in colon tumor cells in mice treated by 1,2-dimethylhydrazine. Resistant starch diet promotes (G) mitophagy and (H) reticulophagy of colon tumor cells in mice treated by 1,2-dimethylhydrazine (magnification, ×40). **P<0.01. SOD, superoxide dismutase; GSH, glutathione synthetase; AMPK, AMP-activated protein kinase; DDIT3, DNA damage-inducible transcript 3 protein; MAP1LC3A, microtubule associated protein 1 light chain 3 α; TOMM20, translocase of outer mitochondrial membrane 20; Neo (Nase), 5′-Nase-ALPase.
Figure 6
Resistant starch diet regulates endoplasmic reticulum stress-dependent PERK activity through upregulation of eIF2α phosphorylation in colon tumor cells. (A) Resistant starch diet increases expression levels of eIF2α, ATF-4 and BACE1 in colon tumor cells. (B) Effects of resistant starch diet on phosphorylation levels of eIF2α and PERK in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (C) Effects of resistant starch diet on activity of eIF2α and PERK in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (D) Resistant starch diet increases expression levels of CHOP, BIP and caspase-12 in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (E) Effects of DReIF2α on PERK activity in colon tumor cells in mice induced by 1,2-dimethylhydrazine. (F) eIF2α knockdown inhibits resistant starch-suppressed ATF-4 and BACE1 expression levels in colon tumor cells in mice fed with resistant starch. (G) Knockdown of eIF2α suppresses expression levels of CHOP, BIP and caspase-12 in colon tumor cells in mice fed with resistant starch. (H) Knockdown of eIF2α inhibits resistant starch-induced apoptosis of colon tumor cells isolated from mice fed with resistant starch. **P<0.01. eIF2α, eukaryotic translation initiation factor 2α; ATF-4, activating transcription factor-4; BACE1, secretase-β; p, phospho; PERK, eukaryotic translation initiation factor 2-α kinase 3; CHOP, DNA damage-inducible transcript 3 protein; BIP, binding immunoglobulin protein; AMPK, AMP-activated protein kinase; DReIF2α, downregulation of eIF2α.
Figure 6
Resistant starch diet regulates endoplasmic reticulum stress-dependent PERK activity through upregulation of eIF2α phosphorylation in colon tumor cells. (A) Resistant starch diet increases expression levels of eIF2α, ATF-4 and BACE1 in colon tumor cells. (B) Effects of resistant starch diet on phosphorylation levels of eIF2α and PERK in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (C) Effects of resistant starch diet on activity of eIF2α and PERK in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (D) Resistant starch diet increases expression levels of CHOP, BIP and caspase-12 in colon tumor cells in mice treated by 1,2-dimethylhydrazine. (E) Effects of DReIF2α on PERK activity in colon tumor cells in mice induced by 1,2-dimethylhydrazine. (F) eIF2α knockdown inhibits resistant starch-suppressed ATF-4 and BACE1 expression levels in colon tumor cells in mice fed with resistant starch. (G) Knockdown of eIF2α suppresses expression levels of CHOP, BIP and caspase-12 in colon tumor cells in mice fed with resistant starch. (H) Knockdown of eIF2α inhibits resistant starch-induced apoptosis of colon tumor cells isolated from mice fed with resistant starch. **P<0.01. eIF2α, eukaryotic translation initiation factor 2α; ATF-4, activating transcription factor-4; BACE1, secretase-β; p, phospho; PERK, eukaryotic translation initiation factor 2-α kinase 3; CHOP, DNA damage-inducible transcript 3 protein; BIP, binding immunoglobulin protein; AMPK, AMP-activated protein kinase; DReIF2α, downregulation of eIF2α.
Similar articles
- Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon.
Bauer-Marinovic M, Florian S, Müller-Schmehl K, Glatt H, Jacobasch G. Bauer-Marinovic M, et al. Carcinogenesis. 2006 Sep;27(9):1849-59. doi: 10.1093/carcin/bgl025. Epub 2006 Apr 5. Carcinogenesis. 2006. PMID: 16597648 - Comparison of resistant starch with cellulose diet on 1,2-dimethylhydrazine-induced colonic carcinogenesis in rats.
Sakamoto J, Nakaji S, Sugawara K, Iwane S, Munakata A. Sakamoto J, et al. Gastroenterology. 1996 Jan;110(1):116-20. doi: 10.1053/gast.1996.v110.pm8536846. Gastroenterology. 1996. PMID: 8536846 - Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids.
Rao CV, Hirose Y, Indranie C, Reddy BS. Rao CV, et al. Cancer Res. 2001 Mar 1;61(5):1927-33. Cancer Res. 2001. PMID: 11280748 - Decoding cell death signals in liver inflammation.
Brenner C, Galluzzi L, Kepp O, Kroemer G. Brenner C, et al. J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review. - The role of carbohydrate fermentation in colon cancer prevention.
Van Munster IP, Nagengast FM. Van Munster IP, et al. Scand J Gastroenterol Suppl. 1993;200:80-6. doi: 10.3109/00365529309101581. Scand J Gastroenterol Suppl. 1993. PMID: 8016578 Review.
Cited by
- Preparation and characterization of resistant starch type III from enzymatically hydrolyzed maize flour.
Khan A, Rahman UU, Siddiqui S, Irfan M, Shah AA, Badshah M, Hasan F, Khan S. Khan A, et al. Mol Biol Rep. 2019 Aug;46(4):4565-4580. doi: 10.1007/s11033-019-04913-5. Epub 2019 Jun 26. Mol Biol Rep. 2019. PMID: 31243724 - Health Effects of Whole Grains: A Bibliometric Analysis.
Wei X, Yang W, Wang J, Zhang Y, Wang Y, Long Y, Tan B, Wan X. Wei X, et al. Foods. 2022 Dec 18;11(24):4094. doi: 10.3390/foods11244094. Foods. 2022. PMID: 36553836 Free PMC article. - Structural Characteristics, Physicochemical Properties, and Digestibility Analysis of Resistant Starch Type-V Prepared from Debranched Corn Starch and Fatty Acid Complexation.
Thakur M, Rai AK, Singh SP. Thakur M, et al. ACS Omega. 2023 Jul 10;8(29):25799-25807. doi: 10.1021/acsomega.3c01093. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521665 Free PMC article. - Screening of Induced Mutants Led to the Identification of Starch Biosynthetic Genes Associated with Improved Resistant Starch in Wheat.
Irshad A, Guo H, Ur Rehman S, Gu J, Wang C, Xiong H, Xie Y, Zhao S, Liu L. Irshad A, et al. Int J Mol Sci. 2022 Sep 15;23(18):10741. doi: 10.3390/ijms231810741. Int J Mol Sci. 2022. PMID: 36142653 Free PMC article. - Current advances in cancer energy metabolism under dietary restriction: a mini review.
Yang L, Shao Y, Gao T, Bajinka O, Yuan X. Yang L, et al. Med Oncol. 2024 Jul 26;41(9):209. doi: 10.1007/s12032-024-02452-z. Med Oncol. 2024. PMID: 39060824 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials