Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons - PubMed (original) (raw)
. 2018 Apr 2;128(4):1657-1670.
doi: 10.1172/JCI94331. Epub 2018 Mar 19.
John K Neubert 2, Danielle M LaPaglia 1, Dragan Maric 3, Jason M Keller 1, Stephen J Raithel 1, Eric L Rohrs 2, Ethan M Anderson 4, John A Butman 5, Robert M Caudle 4, Dorothy C Brown 6, John D Heiss 7, Andrew J Mannes 1, Michael J Iadarola 1
Affiliations
- PMID: 29408808
- PMCID: PMC5873867
- DOI: 10.1172/JCI94331
Pain control through selective chemo-axotomy of centrally projecting TRPV1+ sensory neurons
Matthew R Sapio et al. J Clin Invest. 2018.
Abstract
Agonists of the vanilloid receptor transient vanilloid potential 1 (TRPV1) are emerging as highly efficacious nonopioid analgesics in preclinical studies. These drugs selectively lesion TRPV1+ primary sensory afferents, which are responsible for the transmission of many noxious stimulus modalities. Resiniferatoxin (RTX) is a very potent and selective TRPV1 agonist and is a promising candidate for treating many types of pain. Recent work establishing intrathecal application of RTX for the treatment of pain resulting from advanced cancer has demonstrated profound analgesia in client-owned dogs with osteosarcoma. The present study uses transcriptomics and histochemistry to examine the molecular mechanism of RTX action in rats, in clinical canine subjects, and in 1 human subject with advanced cancer treated for pain using intrathecal RTX. In all 3 species, we observe a strong analgesic action, yet this was accompanied by limited transcriptional alterations at the level of the dorsal root ganglion. Functional and neuroanatomical studies demonstrated that intrathecal RTX largely spares susceptible neuronal perikarya, which remain active peripherally but unable to transmit signals to the spinal cord. The results demonstrate that central chemo-axotomy of the TRPV1+ afferents underlies RTX analgesia and refine the neurobiology underlying effective clinical use of TRPV1 agonists for pain control.
Keywords: Addiction; Clinical Trials; Expression profiling; Neuroscience; Pain.
Conflict of interest statement
Conflict of interest: MJI is an inventor on US patent US 8338457 B2, “Selective ablation of pain-sensing neurons by administration of a vanilloid receptor agonist,” issued to the NIH, and is on the scientific advisory board of Ark Animal Health. JDH has received research support through a cooperative research and development agreement with Sorrento Therapeutics. JKN, ELR, and RMC are employees of Velocity Laboratories, a company that provides fee-for-service behavioral testing using operant pain assays.
Figures
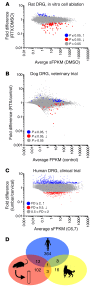
Figure 1. Summary plots of gene changes after RTX treatment.
(A) An in vitro rat study was performed using RTX to ablate TRPV1+ neurons, and RNA-Seq was performed on the remaining cells (n = 3). In this experiment, cell death was confirmed independently. Significant genes (MAGIC pipeline) for this experiment are colored accordingly. (B) Data from 3 control and 5 RTX-treated companion dogs (n = 5 DRGs control, n = 14 DRGs RTX) enrolled in a veterinary trial that were treated intrathecally with RTX are plotted similarly (statistics in limma, voom). (C) A single human patient was treated with RTX intrathecally in the lumbar region. Data from C6,7 are plotted against L5. While some differences may exist between these ganglia, there should also be a gradient of RTX action detectable where RTX acts on the TRPV1+ neurons in the lumbar DRGs more strongly than in the cervical segments. (D) A Venn diagram of genes changing 40% or more shows that there is very little overlap between the genes changing in the 3 studies. Only 1 gene, KLHL1 (Kelch-like family member 1), encoding an actin-organizing protein, changes by at least 40% in all 3 data sets.
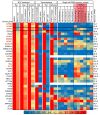
Figure 2. Gene expression map across data sets examining the effects of RTX on gene expression.
Data from 7 experiments were mined and analyzed, including 3 experiments in which the TRPV1 agonist RTX was used to selectively damage TRPV1+ neurons. Genes decreasing by at least 40% are plotted for the 3 experiments in which RTX was used to damage TRPV1+ neurons, and compared with several data sets showing differentials between tissues or cell types to indicate where these genes are expressed. In general, these genes are contributed by neurons, as indicated by their enrichment in DRG versus sciatic nerve, and sorted eTRPV1 neurons versus eIB4+ cells, which includes non-neuronal cells such as microglia and vascular endothelia. Enrichment in TRPV1+ neurons in the single-cell data set was tabulated separately. Modest enrichment in these cells was observed in the rat and dog but not human data sets. Values were normalized such that the highest value in any data set is 1. Data from single cells are normalized differently, according to their original publication. Quantification of enrichment in TRPV1+ and nonpeptidergic 1 populations of cells versus other groups is presented. Genes from rat and dog samples showing a decrease after RTX treatment are enriched in the TRPV1+ cells.
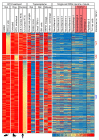
Figure 3. Genes differentially detected in RTX-treated ganglia across experiments in 3 species.
Data from 7 experiments were mined and analyzed, including 3 experiments in which the TRPV1 agonist RTX was used to selectively damage TRPV1+ neurons in the same manner as in the heatmap in Figure 2. Genes were filtered to show only those genes decreasing by at least 20% in human, dog, and rat RTX-treated samples. Genes that are also decreased by at least 30% in all samples are shown in bold orange. The 1 gene (KLHL1) expressed at ≥40% is shown in bold red.
Figure 4. Effects of peripheral versus central targeting of TRPV1 on sensory ganglia.
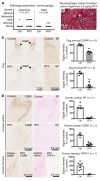
Trigeminal ganglia sections (10 μm, paraffin-embedded) were stained for TRPV1 following 250 ng RTX or vehicle treatment. (A) There was a significant decrease (1-way ANOVA, Scheffé post hoc test, n = 6 sections, n = 3 rats, *P < 0.05) in TRPV1+ cells in the trigeminal ganglia of rats treated with RTX perineurally around the ION as compared with rats treated with the PBS-vehicle. (B) ICM administration of either RTX or PBS-vehicle did not significantly affect the proportion of cells expressing TRPV1. Counts were completed in various regions of the trigeminal ganglia (V1, V2, V3), and the proportion of TRPV1+ cells did not vary significantly. (C) ICM injection of RTX reduced TRPV1 staining in the brainstem and upper cervical spinal cord regions by at least 90% (n = 1). Injection of RTX (250 ng, 10 μl) produced loss of TRPV1+ neurons within the upper cervical region, but there was return of staining at the lower cervical/upper thoracic level and regions more caudal. (D and E) There was a strong reduction in markers of DRG neuronal axons within the brainstem following ICM treatment with RTX (n = 1). Additional quantitation is provided in Supplemental Figure 8. (F–H) Peripheral DRG function was tested by examination of capsaicin-induced plasma extravasation in trigeminally innervated regions of the face after ICM and ION RTX. While ICM RTX completely blocked thermal responses on the ear (G, **P ≤ 0.01, n ≥ 4, ANOVA, Dunnett’s post hoc), it had no effect on capsaicin-induced extravasation (H, n ≥ 3, ANOVA, Dunnett’s post hoc). In contrast, ION injection of RTX significantly blocked extravasation (P ≤ 0.05, n = 5, Student’s t test).
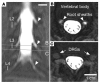
Figure 5. Intrathecal administration of a gadolinium-based contrast agent.
An MRI scan was performed as part of a diagnostic evaluation using a dilute amount of gadolinium-based contrast agent injected intrathecally to trace the distribution of CSF in the lumbar region of a single patient. (A) A coronal maximum-intensity projection of T1-weighted fat-saturated MRI shows a high signal in the thecal sac, which extends along nerve root sheaths (arrowheads), without signal within the lumbar ganglia. (B) Axial sections through the lumbar nerve root sheaths (arrowheads) show contrast filling in the nerve root sheaths approaching the DRGs. (C) Axial sections through the DRG (arrows) show no contrast agent in or around the DRGs. Scale bars: 1 cm.
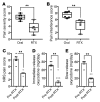
Figure 6. Immunohistochemical staining of DRG and spinal cord from human and canine subjects.
Dog and human sensory ganglia and spinal cord were harvested after intrathecal treatment with RTX, and immunohistochemical staining was performed. Trigeminal and DRG samples from dogs treated with RTX in an independently conducted GLP toxicology study were assessed by a pathologist. (A) At the maximum tolerated dose (3.6 μg/kg), 5 of 10 dogs treated with RTX showed mild pathology in the trigeminal, whereas 3 of 10 showed minimal pathology in the DRG after ICM injection (nonsignificant, Mantel-Haenszel χ2). (B) An example of an infrequent pathological manifestation is the formation of a neuronophagic nodule (arrows). (C) Dogs were injected in the cisterna magna. In the dog, a marked reduction in CGRP was observed in the cervical region of the dorsal spinal cord, with a near-total ablation of substance P (SP) staining at the same level (n = 5 dogs). **P ≤ 0.01. (D) One human patient was injected with RTX using an end-hole catheter positioned at the cauda equina. After RTX treatment, CGRP and SP staining was almost completely abolished in the lumbar spinal cord. Similar reductions were also observed in the thoracic spinal cord for CGRP and TRPV1. A single human was autopsied to procure tissue. To estimate the magnitude of the effect size, several sections of the single human sample (points shown in the graphs) were compared with untreated human sections.
Figure 7. Clinical outcomes in dog and human after RTX treatment.
Dogs injected intrathecally with RTX (n = 6), and the human patient (n = 1) injected intrathecally who was autopsied and whose ganglia were analyzed by RNA-Seq, were assessed for pain control. (A and B) Subsequently to intrathecal RTX treatment, dogs were released to their owners and assessments made at monthly intervals. Owners of each dog rated pain severity and interference with function using the Canine Brief Pain Inventory (78). Pain severity (A) and pain interference (B) scores of companion dogs treated with RTX were significantly decreased relative to those of dogs kept on traditional standard-of-care oral pain medications alone. (C–E) Data from 1 human patient were collected over 7 days before RTX and 15 days after RTX injection while he was an inpatient in the oncology ward. Beginning immediately after RTX treatment, Numerical Rating Scale (NRS) pain scores (1 to 10) decreased from about 8 to about 4 (C). Concurrently, self-administration of immediate-release oxycodone was decreased (D). Slow-release oxycodone was also reduced during the same period (E). Statistical comparisons were made using a 2-tailed Mann-Whitney U test (**P < 0.01).
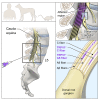
Figure 8. Suggested model of RTX mechanism in rodent, canine, and human.
RTX acts at the level of the spinal cord to ablate only the centrally projecting axon of TRPV1-expressing C and Aδ neurons. The neuronal cell bodies in the DRG and the peripherally projecting axonal portion of the neurons are not bathed in drug-containing CSF and remain intact and functional. The TRPV1+ axons are only a subpopulation of the somatosensory and proprioceptive afferent populations. Only the TRPV1+ population is susceptible.
Similar articles
- Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia.
Chen SR, Pan HL. Chen SR, et al. J Neurophysiol. 2006 May;95(5):3086-96. doi: 10.1152/jn.01343.2005. Epub 2006 Feb 8. J Neurophysiol. 2006. PMID: 16467418 - Removing TRPV1-expressing primary afferent neurons potentiates the spinal analgesic effect of delta-opioid agonists on mechano-nociception.
Chen SR, Pan HL. Chen SR, et al. Neuropharmacology. 2008 Aug;55(2):215-22. doi: 10.1016/j.neuropharm.2008.05.011. Epub 2008 May 22. Neuropharmacology. 2008. PMID: 18579164 Free PMC article. - Supraspinal-selective TRPV1 desensitization induced by intracerebroventricular treatment with resiniferatoxin.
Fukushima A, Mamada K, Iimura A, Ono H. Fukushima A, et al. Sci Rep. 2017 Sep 29;7(1):12452. doi: 10.1038/s41598-017-12717-5. Sci Rep. 2017. PMID: 28963471 Free PMC article. - The vanilloid agonist resiniferatoxin for interventional-based pain control.
Iadarola MJ, Mannes AJ. Iadarola MJ, et al. Curr Top Med Chem. 2011;11(17):2171-9. doi: 10.2174/156802611796904942. Curr Top Med Chem. 2011. PMID: 21671877 Free PMC article. Review. - Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity.
Kissin I. Kissin I. Anesth Analg. 2008 Jul;107(1):271-81. doi: 10.1213/ane.0b013e318162cfa3. Anesth Analg. 2008. PMID: 18635498 Free PMC article. Review.
Cited by
- Peripheral mechanisms of arthritic pain: A proposal to leverage large animals for in vitro studies.
Chakrabarti S, Ai M, Henson FMD, Smith ESJ. Chakrabarti S, et al. Neurobiol Pain. 2020 Jul 28;8:100051. doi: 10.1016/j.ynpai.2020.100051. eCollection 2020 Aug-Dec. Neurobiol Pain. 2020. PMID: 32817908 Free PMC article. Review. - Molecular Pathways Linking Oxylipins to Nociception in Rats.
Domenichiello AF, Sapio MR, Loydpierson AJ, Maric D, Goto T, Horowitz MS, Keyes GS, Yuan ZX, Majchrzak-Hong SF, Mannes AJ, Iadarola MJ, Ramsden CE. Domenichiello AF, et al. J Pain. 2021 Mar;22(3):275-299. doi: 10.1016/j.jpain.2020.09.001. Epub 2020 Oct 6. J Pain. 2021. PMID: 33031942 Free PMC article. - Be in it for the Long Haul: A Commentary on Human Tissue Recovery Initiatives.
Iadarola MJ, Sapio MR, Mannes AJ. Iadarola MJ, et al. J Pain. 2022 Oct;23(10):1646-1650. doi: 10.1016/j.jpain.2022.04.009. Epub 2022 Apr 30. J Pain. 2022. PMID: 35504570 Free PMC article. - Proteomics Reveals Long-Term Alterations in Signaling and Metabolic Pathways Following Both Myocardial Infarction and Chemically Induced Denervation.
Salem JB, Iacovoni JS, Calise D, Arvanitis DN, Beaudry F. Salem JB, et al. Neurochem Res. 2022 Aug;47(8):2416-2430. doi: 10.1007/s11064-022-03636-7. Epub 2022 Jun 18. Neurochem Res. 2022. PMID: 35716295 - Study on mechanism of transdermal administration of eugenol for pain treatment by network pharmacology and molecular docking technology.
Ye H, Lin Q, Mei Q, Liu Q, Cao S. Ye H, et al. Heliyon. 2024 Apr 16;10(8):e29722. doi: 10.1016/j.heliyon.2024.e29722. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681628 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- K08 DA017720/DA/NIDA NIH HHS/United States
- K22 DE014865/DE/NIDCR NIH HHS/United States
- R21 DE016704/DE/NIDCR NIH HHS/United States
- ZIA AT000017/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical