Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy - PubMed (original) (raw)
. 2018 Feb 7;13(2):e0191290.
doi: 10.1371/journal.pone.0191290. eCollection 2018.
Jaeyeol Kwon 1, Seohyun Park 1, Jong Hyun Jhee 1, Hae-Ryong Yun 1, HyoungNae Kim 1, Youn Kyung Kee 1, Chang-Yun Yoon 1, Tae-Ik Chang 2, Ea Wha Kang 2, Jung Tak Park 1, Tae-Hyun Yoo 1, Shin-Wook Kang 1, Seung Hyeok Han 1
Affiliations
- PMID: 29415048
- PMCID: PMC5802883
- DOI: 10.1371/journal.pone.0191290
Phosphate is a potential biomarker of disease severity and predicts adverse outcomes in acute kidney injury patients undergoing continuous renal replacement therapy
Su-Young Jung et al. PLoS One. 2018.
Abstract
Hyperphosphatemia is associated with mortality in patients with chronic kidney disease, and is common in critically ill patients with acute kidney injury (AKI); however, its clinical implication in these patients is unknown. We conducted an observational study in 1144 patients (mean age, 63.2 years; male, 705 [61.6%]) with AKI who received continuous renal replacement therapy (CRRT) between January 2009 and September 2016. Phosphate levels were measured before (0 h) and 24 h after CRRT initiation. We assessed disease severity using various clinical parameters. Phosphate at 0 h positively correlated with the Acute Physiology and Chronic Health Evaluation II (APACHE II; P < 0.001) and Sequential Organ Failure Assessment (SOFA; P < 0.001) scores, and inversely with mean arterial pressure (MAP; P = 0.02) and urine output (UO; P = 0.01). In a fully adjusted linear regression analysis for age, sex, Charlson comorbidity index (CCI), MAP, and estimated glomerular filtration rate (eGFR), higher 0 h phosphate level was significantly associated with high APACHE II (P < 0.001) and SOFA (P = 0.04) scores, suggesting that phosphate represents disease severity. A multivariable Cox model also showed that hyperphosphatemia was significantly associated with increased 28-day (HR 1.05, 95% CI 1.02-1.08, P = 0.001) and 90-day (HR 1.05, 95% CI 1.02-1.08, P = 0.001) mortality. Furthermore, patients with increased phosphate level during 24 h were at higher risk of death than those with stable or decreased phosphate levels. Finally, c-statistics significantly increased when phosphate was added to a model that included age, sex, CCI, body mass index, eGFR, MAP, hemoglobin, serum albumin, C-reactive protein, and APACHE II score. This study shows that phosphate is a potential biomarker that can reflect disease severity and predict mortality in critically ill patients receiving CRRT.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Fig 1. Flow chart of patient selection.
CRRT continuous renal replacement therapy, AKIN acute kidney injury network, CKD chronic kidney disease.
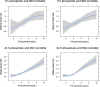
Fig 2. Cubic spline analysis of the associations between phosphate levels and 28- and 90-day mortality.
Hazard ratios (HRs) were adjusted for age, sex, body mass index (BMI), Charlson comorbidity index (CCI), Sequential Organ Failure Assessment (SOFA) score, urine output (UO), albumin, and mean arterial pressure (MAP). Line represents HR. Shaded area represents 95% CI for the HR.
Fig 3. Kaplan-Meier plots for 28- and 90-day mortality according to phosphate change.
Group 1 (phosphate decrease group), ≥-1.3 mg/dL decrease; group 2 (stable group), -1.3 to 0 mg/dL decrease; group 3 (phosphate increase group).
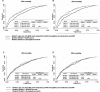
Fig 4. Receiver-operating characteristic plots representing the area under the curve (AUC) for the prediction of 28- and 90-daay mortality according to 0 h phosphate.
The AUCs for 28- (A) and 90- day (B) mortality using models with SOFA score; The AUCs for 28- (C) and 90- day (D) mortality using models with APACHE II score. Comparison P values were calculated.
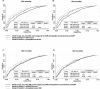
Fig 5. Receiver-operating characteristic plots representing the area under the curve (AUC) for the prediction of 28- and 90-day mortality according to 24 h phosphate.
The AUCs for 28- (A) and 90- day (B) mortality using models with SOFA score; The AUCs for 28- (C) and 90- day (D) mortality using models with APACHE II score. Comparison P values were calculated.
Similar articles
- Urine output is associated with prognosis in patients with acute kidney injury requiring continuous renal replacement therapy.
Oh HJ, Shin DH, Lee MJ, Ko KI, Kim CH, Koo HM, Doh FM, Kwon YE, Kim YL, Nam KH, Park KS, An SY, Park JT, Han SH, Yoo TH, Kang SW. Oh HJ, et al. J Crit Care. 2013 Aug;28(4):379-88. doi: 10.1016/j.jcrc.2012.11.019. Epub 2013 Apr 10. J Crit Care. 2013. PMID: 23582311 - SOFA score is superior to APACHE-II score in predicting the prognosis of critically ill patients with acute kidney injury undergoing continuous renal replacement therapy.
Wang H, Kang X, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH. Wang H, et al. Ren Fail. 2020 Nov;42(1):638-645. doi: 10.1080/0886022X.2020.1788581. Ren Fail. 2020. PMID: 32660294 Free PMC article. - Combination of Mean Platelet Volume/Platelet Count Ratio and the APACHE II Score Better Predicts the Short-Term Outcome in Patients with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy.
Li J, Li Y, Sheng X, Wang F, Cheng D, Jian G, Li Y, Feng L, Wang N. Li J, et al. Kidney Blood Press Res. 2018;43(2):479-489. doi: 10.1159/000488694. Epub 2018 Mar 29. Kidney Blood Press Res. 2018. PMID: 29627837 - Mean platelet volume is a prognostic factor in patients with acute kidney injury requiring continuous renal replacement therapy.
Han JS, Park KS, Lee MJ, Kim CH, Koo HM, Doh FM, Kim EJ, Han JH, Park JT, Han SH, Yoo TH, Kang SW, Oh HJ. Han JS, et al. J Crit Care. 2014 Dec;29(6):1016-21. doi: 10.1016/j.jcrc.2014.07.022. Epub 2014 Jul 24. J Crit Care. 2014. PMID: 25138689 - The impact of disease severity on paradoxical association between body mass index and mortality in patients with acute kidney injury undergoing continuous renal replacement therapy.
Kim H, Kim H, Lee M, Cha MU, Nam KH, An SY, Jung SY, Jhee JH, Park S, Yun HR, Kee YK, Oh HJ, Park JT, Chang TI, Yoo TH, Kang SW, Han SH. Kim H, et al. BMC Nephrol. 2018 Feb 7;19(1):32. doi: 10.1186/s12882-018-0833-5. BMC Nephrol. 2018. PMID: 29415663 Free PMC article.
Cited by
- Transfer learning-enabled outcome prediction for guiding CRRT treatment of the pediatric patients with sepsis.
Li XQ, Wang RQ, Wu LQ, Chen DM. Li XQ, et al. BMC Med Inform Decis Mak. 2024 Sep 27;24(1):266. doi: 10.1186/s12911-024-02623-y. BMC Med Inform Decis Mak. 2024. PMID: 39334261 Free PMC article. - Higher body mass index is not a protective risk factor for 28-days mortality in critically ill patients with acute kidney injury undergoing continuous renal replacement therapy.
Wang H, Shi Y, Bai ZH, Lv JH, Sun JL, Pei HH, Zhang ZL. Wang H, et al. Ren Fail. 2019 Nov;41(1):726-732. doi: 10.1080/0886022X.2019.1650767. Ren Fail. 2019. PMID: 31424314 Free PMC article. - A novel method for automated crystal visualization and quantification in murine folic acid-induced acute kidney injury.
Hamid AK, Pastor Arroyo EM, Lee SS, Wagner CA, Egli-Spichtig D. Hamid AK, et al. Am J Physiol Renal Physiol. 2024 Jan 1;326(1):F105-F117. doi: 10.1152/ajprenal.00140.2023. Epub 2023 Oct 26. Am J Physiol Renal Physiol. 2024. PMID: 37881875 Free PMC article. - Using machine learning to predict the risk of short-term and long-term death in acute kidney injury patients after commencing CRRT.
Gu M, Liu Y, Sun H, Sun H, Fang Y, Chen L, Zhang L. Gu M, et al. BMC Nephrol. 2024 Jul 30;25(1):245. doi: 10.1186/s12882-024-03676-x. BMC Nephrol. 2024. PMID: 39080581 Free PMC article.
References
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8. doi: 10.1001/jama.294.7.813 . - DOI - PubMed
- Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–24. doi: 10.1038/ki.2012.208 . - DOI - PubMed
- Investigators RRTS, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–38. doi: 10.1056/NEJMoa0902413 . - DOI - PubMed
- Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016;375(2):122–33. doi: 10.1056/NEJMoa1603017 . - DOI - PubMed
- Demirjian S, Chertow GM, Zhang JH, O'Connor TZ, Vitale J, Paganini EP, et al. Model to predict mortality in critically ill adults with acute kidney injury. Clin J Am Soc Nephrol. 2011;6(9):2114–20. doi: 10.2215/CJN.02900311 ; PubMed Central PMCID: PMCPMC3359007. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
The authors received no specific funding for this work.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous