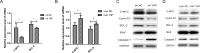C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC - PubMed (original) (raw)
C-MYC and BCL-2 mediate YAP-regulated tumorigenesis in OSCC
Xiyan Chen et al. Oncotarget. 2017.
Abstract
Transcriptional co-activator Yes-associated protein (YAP) is a key oncogene in mammalian cells. The present understanding of YAP in oral squamous cells carcinoma (OSCC) remains unclear. The purpose of this study is to investigate the effects of YAP on proliferation and apoptosis in OSCC and the molecular mechanism. The results showed the expression level of YAP was higher in OSCC tissues than that in adjacent normal tissues. Knockdown of YAP in CAL27 cell lines prohibited cell proliferation while augmented apoptosis. Conversely, overexpression of YAP protected cells from apoptosis and promoted cell proliferation. Moreover, C-MYC and BCL-2 mRNA and protein levels were altered due to the differential expression of YAP. Subsequent Verteporfin treatment in CAL27 cells revealed that the transcription and translation of BCL-2 and C-MYC both decreased. In a tumor xenograft model, knockdown of YAP suppressed tumor growth of CAL27 in vivo, while YAP overexpression promoted the tumor growth. These results suggest that YAP is a crucial regulator that exerts pro-proliferation and anti-apoptosis effects in OSCC through actions affecting the cell cycle and intrinsic apoptotic signaling. Thus YAP could potentially serve as a valuable molecular biomarker or therapeutic target in the treatment of OSCC.
Keywords: BCL-2; C-MYC; oral squamous cell carcinoma (OSCC); tumorigenesis; yes-associated protein(YAP).
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no conflicts of interest.
Figures
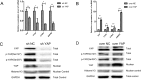
Figure 1. YAP was highly expressed in oral squamous cell carcinoma
(A) Representative images of YAP immunostaining in normal tissues, well differentiated OSCC tissues, moderately differentiated OSCC tissues and poorly differentiated OSCC tissues. Low power (100×) scale bars 100 μm, high power (200×) scale bars: 50 μm. (B) Statistical relative quantification of YAP expression. (C) Western blot analysis of expression of YAP in four OSCC cell lines. (D) RT-PCR analysis of YAP expression in four OSCC cell lines. *p < 0.05, **P < 0.01, ***p < 0.001.
Figure 2. Knockdown and overexpression of YAP were generated in CAL27 cells
(A, B) RT- PCR was conducted to determine the mRNA levels of YAP and the downstream genes of Hippo pathway in CAL27 cells. (C, D) CAL27 cells with overexpression or knockdown of YAP were used to analyze the expression of YAP, p-YAP (Ser127), p-YAP (Ser397). GAPDH was used as a total control and Histone H3 was used as a nuclear control. *p < 0.05, **P < 0.01, ***p < 0.001.
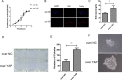
Figure 3. Knockdown of YAP inhibited cells proliferation
(A) Cell proliferation was detected in CAL27 cells by CCK-8 assays. (B) The proliferating CAL27 cells were labeled with EdU. The images were representative of the results obtained. Scale bar: 50 μm. (C) Numbers of EdU-positive CAL27 cells. Shown is the proportion of EdU-positive CAL27 cells to all nuclei. (D) Colony formation assays of CAL27 cells. (E) Statically analysis of colony formation assay. The colonies consisting of more than 50 cells were counted. (F) Images of single colony of CAL27 cells. *p < 0.05, **P < 0.01, ***p < 0.001.
Figure 4. YAP overexpression promoted cells proliferation
(A) The proliferation of CAL27 cells was measured by CCK-8 assay. (B) YAP overexpression resulted in increased CAL27 cells proliferation. Shown are representative images of the EdU staining. Scale bar: 50 μm. (C) Percent of EdU positive cells in CAL27 cells. (D) The effects of YAP upregulated on the colony formation of CAL27 cells. (E) Numbers of colonies. Data were listed as mean ± SD of three wells. (F) The effects of YAP overexpression on single colony. *p < 0.05, **P < 0.01, ***p < 0.001.
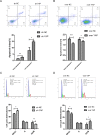
Figure 5. The effects of YAP knockdown or overexpression on cell apoptosis and cell cycle transition
(A, B) The cell cycle phase distribution was evaluated by FACS. Shown are representative images of separate experiments. The cell cycle distribution was calculated and expressed as mean ± SD of 3 separate experiments. (C, D) CAL27 cells apoptosis were measured by Annexin-V-APC/7AAD staining. Statistical analysis of flow cytometry results. The presented columns are given as the means ± SD. *p < 0.05, **P < 0.01, ***p < 0.001.
Figure 6. C-MYC and BCL-2 were the target genes of YAP-TEAD1 transcriptional active complex
(A, B) The changes of mRNA levels of C-MYC and BCL-2 after YAP knockdown or overexpression. (C, D) Western blot analysis of the expression of C-MYC and BCL-2 and other cell cycle-related and apoptosis-related proteins, such as Cyclin D1, BAX, Caspase7. GAPDH was used as a loading control. *p < 0.05.
Figure 7. Verteporfin treatment inhibited the transcription of BCL-2 and C-MYC
(A) Verteporfin inhibited the proliferation activity of CAL27 cell lines. (B) The cell cycle of CAL27 cells treated with Verteporfin arrested in S phase. (C) The proportion of early and late apoptosis cells increased after Verteporfin treatment. (D) The mRNA expression level of BCL-2 and C-MYC decreased after Verteporfin treatment, along with YAP target gene CTGF and CYR61. (E) The expression of BCL-2 and C-MYC proteins decreased with the increase of Verteporfin concentration. The presented columns are given as the means ± SD. *p < 0.05, **P < 0.01, ***p < 0.001.
Figure 8. YAP accelerated tumorigenicity of OSCC cells in vivo
(A, D) Time course analysis of tumor growth in nude mice after injection of CAL27 cells. (B, E) The tumors weight were weighed and analyzed by Student t test. Means ± SD were shown. (C, F) Representative images (week 4) of subcutaneous tumor xenograft in nude mice inoculated with CAL27 cells. *p < 0.05, **P < 0.01, ***p < 0.001.
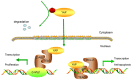
Figure 9. Hypothetical model of YAP-regulated tumorigenesis in OSCC
Inactivated of Hippo signal pathway determines an increase of YAP and its nuclear translocation. YAP could indirectly or directly drive C-MYC transcriptional activity, which leads to sustained cell proliferation. Meanwhile, YAP is able to increase the transcription of BCL-2 gene and result in resisting cell apoptosis.
Similar articles
- Metformin inhibits mTOR and c-Myc by decreasing YAP protein expression in OSCC cells.
Wang Y, Zhang Y, Feng X, Tian H, Fu X, Gu W, Wen Y. Wang Y, et al. Oncol Rep. 2021 Mar;45(3):1249-1260. doi: 10.3892/or.2020.7909. Epub 2020 Dec 24. Oncol Rep. 2021. PMID: 33650651 - Yes-associated protein promotes cell migration via activating Wiskott-Aldrich syndrome protein family member 1 in oral squamous cell carcinoma.
Qi L, Shi C, Li J, Xu S, Han Y, Li J, Zhang L. Qi L, et al. J Oral Pathol Med. 2019 Apr;48(4):290-298. doi: 10.1111/jop.12833. Epub 2019 Feb 19. J Oral Pathol Med. 2019. PMID: 30697796 - Yes-associated protein promotes cell proliferation by activating Fos Related Activator-1 in oral squamous cell carcinoma.
Zhang L, Ye DX, Pan HY, Wei KJ, Wang LZ, Wang XD, Shen GF, Zhang ZY. Zhang L, et al. Oral Oncol. 2011 Aug;47(8):693-7. doi: 10.1016/j.oraloncology.2011.06.003. Epub 2011 Jun 25. Oral Oncol. 2011. PMID: 21708480 - Induction of apoptosis using ATN as a novel Yes-associated protein inhibitor in human oral squamous cell carcinoma cells.
Chu PC, Dokla EME, Hu JL, Weng JR. Chu PC, et al. Environ Toxicol. 2022 Jun;37(6):1404-1412. doi: 10.1002/tox.23493. Epub 2022 Feb 25. Environ Toxicol. 2022. PMID: 35212453 - YAP at the progression of inflammation.
Chen L, Jin X, Ma J, Xiang B, Li X. Chen L, et al. Front Cell Dev Biol. 2023 Jun 15;11:1204033. doi: 10.3389/fcell.2023.1204033. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37397250 Free PMC article. Review.
Cited by
- Rab14 overexpression regulates gemcitabine sensitivity through regulation of Bcl-2 and mitochondrial function in pancreatic cancer.
Ge J, Ge C. Ge J, et al. Virchows Arch. 2019 Jan;474(1):59-69. doi: 10.1007/s00428-018-2455-5. Epub 2018 Sep 29. Virchows Arch. 2019. PMID: 30267303 - Prolactin promotes crop epithelial proliferation of domestic pigeons (Columba livia) through the Hippo signaling pathway.
Zhu J, Teng X, Wang L, Zheng M, Meng Y, Liu T, Liu Y, Huan H, Gong D, Xie P. Zhu J, et al. J Anim Sci. 2023 Jan 3;101:skad312. doi: 10.1093/jas/skad312. J Anim Sci. 2023. PMID: 37721785 Free PMC article. - A New Player in Neuroblastoma: YAP and Its Role in the Neuroblastoma Microenvironment.
Shim J, Goldsmith KC. Shim J, et al. Cancers (Basel). 2021 Sep 16;13(18):4650. doi: 10.3390/cancers13184650. Cancers (Basel). 2021. PMID: 34572875 Free PMC article. Review. - Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro†.
Plewes MR, Hou X, Zhang P, Liang A, Hua G, Wood JR, Cupp AS, Lv X, Wang C, Davis JS. Plewes MR, et al. Biol Reprod. 2019 Nov 21;101(5):1001-1017. doi: 10.1093/biolre/ioz139. Biol Reprod. 2019. PMID: 31350850 Free PMC article. - CircZDBF2 up-regulates RNF145 by ceRNA model and recruits CEBPB to accelerate oral squamous cell carcinoma progression via NFκB signaling pathway.
Rong L, Chen B, Liu K, Liu B, He X, Liu J, Li J, He M, Zhu L, Liu K, Shi X, Shuai Y, Jin L. Rong L, et al. J Transl Med. 2022 Apr 1;20(1):148. doi: 10.1186/s12967-022-03347-1. J Transl Med. 2022. PMID: 35365168 Free PMC article.
References
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. - PubMed
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma—an update. CA Cancer J Clin. 2015;65:401–21. - PubMed
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. - PubMed
- Guo L, Teng L. YAP/TAZ for cancer therapy: opportunities and challenges (review) Int J Oncol. 2015;46:1444–52. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources