Value of Circulating Cytokine Profiling During Submaximal Exercise Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - PubMed (original) (raw)
Value of Circulating Cytokine Profiling During Submaximal Exercise Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Kegan J Moneghetti et al. Sci Rep. 2018.
Abstract
Myalgic Encephalomyelitis or Chronic Fatigue Syndrome (ME/CFS) is a heterogeneous syndrome in which patients often experience severe fatigue and malaise following exertion. Immune and cardiovascular dysfunction have been postulated to play a role in the pathophysiology. We therefore, examined whether cytokine profiling or cardiovascular testing following exercise would differentiate patients with ME/CFS. Twenty-four ME/CFS patients were matched to 24 sedentary controls and underwent cardiovascular and circulating immune profiling. Cardiovascular analysis included echocardiography, cardiopulmonary exercise and endothelial function testing. Cytokine and growth factor profiles were analyzed using a 51-plex Luminex bead kit at baseline and 18 hours following exercise. Cardiac structure and exercise capacity were similar between groups. Sparse partial least square discriminant analyses of cytokine profiles 18 hours post exercise offered the most reliable discrimination between ME/CFS and controls (κ = 0.62(0.34,0.84)). The most discriminatory cytokines post exercise were CD40L, platelet activator inhibitor, interleukin 1-β, interferon-α and CXCL1. In conclusion, cytokine profiling following exercise may help differentiate patients with ME/CFS from sedentary controls.
Conflict of interest statement
The authors declare no competing interests.
Figures
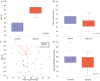
Figure 1
Clinical demographics of Controls and ME/CFS. There was a significant difference in MFI-20 between controls and participants with ME/CFS (A). Groups were similar with regard to maximal oxygen consumption (peak VO2) (B), however, MFI-20 did not correlate to peak VO2 (C). There was a reduction in absolute heart rate recovery slope in patients with ME/CFS when compared to controls (D).
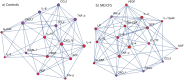
Figure 2
Network of the change in cytokines with exercise. Vertex colors are dark blue for strongly negative average delta to dark red for strongly positive average delta. Degree of centrality of cytokines is represented by the diameter of its vertex. The diameter of a cytokine’s vertex is proportional to its quantity of direct direct connections (blue lines) with other cytokines. In (A) Controls: IL-5, TNF-alpha and IL-2 are richly connected while in (B) ME/CFS: CXCL10, VEGF and IL-15 are richly interconnected. IL-4 appeared to play a similar role in both networks.
Figure 3
Partial Least Square discriminant analysis to identify factors associated with ME/CFS case status. Kappa (κ) is coefficient of chance-adjusted agreement, here between observed case status and predicted case status from sPLSDA. Cross-validated discriminatory cytokines are those above the horizontal dashed red line. At Baseline (A) 18 hours post exercise (B) and delta change between A and B (C) Summary of sPLSDA with κ coefficients (D).
Similar articles
- Whole blood human transcriptome and virome analysis of ME/CFS patients experiencing post-exertional malaise following cardiopulmonary exercise testing.
Bouquet J, Li T, Gardy JL, Kang X, Stevens S, Stevens J, VanNess M, Snell C, Potts J, Miller RR, Morshed M, McCabe M, Parker S, Uyaguari M, Tang P, Steiner T, Chan WS, De Souza AM, Mattman A, Patrick DM, Chiu CY. Bouquet J, et al. PLoS One. 2019 Mar 21;14(3):e0212193. doi: 10.1371/journal.pone.0212193. eCollection 2019. PLoS One. 2019. PMID: 30897114 Free PMC article. - Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
Shukla SK, Cook D, Meyer J, Vernon SD, Le T, Clevidence D, Robertson CE, Schrodi SJ, Yale S, Frank DN. Shukla SK, et al. PLoS One. 2015 Dec 18;10(12):e0145453. doi: 10.1371/journal.pone.0145453. eCollection 2015. PLoS One. 2015. PMID: 26683192 Free PMC article. - Cytokine responses to exercise and activity in patients with chronic fatigue syndrome: case-control study.
Clark LV, Buckland M, Murphy G, Taylor N, Vleck V, Mein C, Wozniak E, Smuk M, White PD. Clark LV, et al. Clin Exp Immunol. 2017 Dec;190(3):360-371. doi: 10.1111/cei.13023. Epub 2017 Oct 11. Clin Exp Immunol. 2017. PMID: 28779554 Free PMC article. Clinical Trial. - A systematic review of cytokines in chronic fatigue syndrome/myalgic encephalomyelitis/systemic exertion intolerance disease (CFS/ME/SEID).
Corbitt M, Eaton-Fitch N, Staines D, Cabanas H, Marshall-Gradisnik S. Corbitt M, et al. BMC Neurol. 2019 Aug 24;19(1):207. doi: 10.1186/s12883-019-1433-0. BMC Neurol. 2019. PMID: 31445522 Free PMC article. - Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic literature review.
Nijs J, Nees A, Paul L, De Kooning M, Ickmans K, Meeus M, Van Oosterwijck J. Nijs J, et al. Exerc Immunol Rev. 2014;20:94-116. Exerc Immunol Rev. 2014. PMID: 24974723 Review.
Cited by
- Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome.
Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, Grabowski P, Doehner W, Scheibenbogen C. Scherbakov N, et al. ESC Heart Fail. 2020 Jun;7(3):1064-1071. doi: 10.1002/ehf2.12633. Epub 2020 Mar 10. ESC Heart Fail. 2020. PMID: 32154656 Free PMC article. - Cardiopulmonary, metabolic, and perceptual responses during exercise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Multi-site Clinical Assessment of ME/CFS (MCAM) sub-study.
Cook DB, VanRiper S, Dougherty RJ, Lindheimer JB, Falvo MJ, Chen Y, Lin JS, Unger ER; MCAM Study Group. Cook DB, et al. PLoS One. 2022 Mar 15;17(3):e0265315. doi: 10.1371/journal.pone.0265315. eCollection 2022. PLoS One. 2022. PMID: 35290404 Free PMC article. - Recent research in myalgic encephalomyelitis/chronic fatigue syndrome: an evidence map.
Todhunter-Brown A, Campbell P, Broderick C, Cowie J, Davis B, Fenton C, Markham S, Sellers C, Thomson K; NIHR Evidence Synthesis Scotland Initiative (NESSIE). Todhunter-Brown A, et al. Health Technol Assess. 2025 Mar 26:1-78. doi: 10.3310/BTBD8846. Online ahead of print. Health Technol Assess. 2025. PMID: 40162526 Free PMC article. - Cell-Based Blood Biomarkers for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.
Missailidis D, Sanislav O, Allan CY, Annesley SJ, Fisher PR. Missailidis D, et al. Int J Mol Sci. 2020 Feb 8;21(3):1142. doi: 10.3390/ijms21031142. Int J Mol Sci. 2020. PMID: 32046336 Free PMC article. - Sex-Dependent Transcriptional Changes in Response to Stress in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Pilot Project.
Gamer J, Van Booven DJ, Zarnowski O, Arango S, Elias M, Kurian A, Joseph A, Perez M, Collado F, Klimas N, Oltra E, Nathanson L. Gamer J, et al. Int J Mol Sci. 2023 Jun 17;24(12):10255. doi: 10.3390/ijms241210255. Int J Mol Sci. 2023. PMID: 37373402 Free PMC article.
References
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue, S., Board on the Health of Select, P. & Institute of, M. In Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illnesswww.nap.edu/download/19012 (2015) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical