Nicotine-Use/Smoking Is Associated with the Efficacy of Naltrexone in the Treatment of Alcohol Dependence - PubMed (original) (raw)
Randomized Controlled Trial
. 2018 Apr;42(4):751-760.
doi: 10.1111/acer.13601. Epub 2018 Feb 12.
Affiliations
- PMID: 29431852
- PMCID: PMC5880727
- DOI: 10.1111/acer.13601
Randomized Controlled Trial
Nicotine-Use/Smoking Is Associated with the Efficacy of Naltrexone in the Treatment of Alcohol Dependence
Raymond F Anton et al. Alcohol Clin Exp Res. 2018 Apr.
Abstract
Background: The opioid antagonist naltrexone is not efficacious for every alcohol treatment seeker. However, various individual factors, such as genetic differences and nicotine-use/smoking status, have been suggested as predictors of naltrexone response. In a randomized clinical trial, we previously reported that nicotine-use/smoking status might be a stronger predictor of naltrexone efficacy than OPRM1 A118G single nucleotide polymorphism (SNP) genotype. In this report, we further characterize the nicotine-users in that trial, examine other drinking outcomes, examine the influence of smoking change on naltrexone effects on drinking, and validate the result in smokers with disialo carbohydrate-deficient transferrin (%dCDT) change as an independent biomarker of response.
Methods: Individuals (n = 146) meeting DSM-IV criteria for alcohol dependence who were genotyped for the OPRM1 A118G SNP and who did, or did not, use nicotine/cigarettes were randomized, in a balanced fashion, to naltrexone (50 mg/d) or placebo and provided medical management (MM) over a 16-week clinical trial. Alcohol use and smoking during the trial were assessed and analyzed.
Results: Nicotine-use/smoking status significantly interacted with medication in reducing percent heavy drinking days (PHDD) during the trial (p = 0.003), such that nicotine-users/smokers showed significantly lower PHDD on naltrexone versus placebo (p = 0.0001, Cohen's d = 0.89), while nonusers showed no significant difference between naltrexone and placebo (p = 0.95, Cohen's d = 0.02). Similar effects were shown for drinks per day and percent days drinking. The superiority of naltrexone over placebo on PHDD reduction in nicotine-users/smokers was confirmed with %dCDT (Cohen's d range 0.3 to 0.9 over the study). Naltrexone did not significantly change cigarette use in smokers, and change in use did not influence naltrexone's effect on PHDD.
Conclusions: These data confirm past findings that naltrexone is more efficacious in those who use nicotine/cigarettes. Compared to previous work on the OPRM1 A118G SNP, it appears that nicotine-use might be a more salient predictor of naltrexone treatment response. While naltrexone did not change cigarette use during the study, and smoking change was not related to alcohol reduction, it should be noted that participants were not seeking smoking cessation and MM did not address this issue.
Keywords: Alcohol; Alcohol Use Disorder; Naltrexone; Nicotine-Use; Treatment.
Copyright © 2018 by the Research Society on Alcoholism.
Conflict of interest statement
All other authors report no biomedical conflicts of interest.
Figures
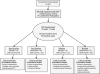
Figure 1
Consort diagram of study design and recruitment and randomization.
Figure 2
Percent heavy drinking days (means +/− SEM) during the study in non-nicotine and nicotine users treated with naltrexone or placebo. * significant interaction of nicotine-use by medication group (p = 0.003). ** significant difference in naltrexone vs. placebo in nicotine users (p = 0.0001).
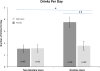
Figure 3
Drinks per day (means +/− SEM) during the study in non-nicotine and nicotine users treated with naltrexone or placebo. * significant interaction of nicotine-use by medication group (p = 0.008). ** significant difference in naltrexone vs. placebo in nicotine users (p = 0.0001).
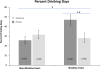
Figure 4
Percent drinking days (means +/− SEM) during the study in non-nicotine and nicotine users treated with naltrexone or placebo. * significant interaction of nicotine-use by medication group (p = 0.015). ** significant difference in naltrexone vs. placebo in nicotine users (p = 0.016).
Figure 5
A) Percent heavy drinking days (means +/− SEM) over the course of the study in nicotine users treated with naltrexone or placebo. B) Parallel change in %dCDT, a blood marker of heavy drinking, in the same individuals over the course of the study. Effect sizes (Cohen’s d) and significance is provided for the naltrexone vs. placebo contrast at various study weeks for both verbally reported heavy drinking days and %dCDT blood marker of heavy drinking days.
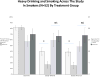
Figure 6
Means (SEM) of percent heavy drinking days (left axis and stippled color) and cigarettes per smoking day (right axis and solid color) in 52 smokers over the course of the study in those treated with naltrexone or placebo. Naltrexone treatment led to significantly less PHDD than placebo at both week 8 (p=0.02) and week 16 (p=0.02) of treatment. There was no significant difference between naltrexone or placebo in cigarette use at either week 8 or week 16.
Similar articles
- Predictors of Naltrexone Response in a Randomized Trial: Reward-Related Brain Activation, OPRM1 Genotype, and Smoking Status.
Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF. Schacht JP, et al. Neuropsychopharmacology. 2017 Dec;42(13):2640-2653. doi: 10.1038/npp.2017.74. Epub 2017 Apr 14. Neuropsychopharmacology. 2017. PMID: 28409564 Free PMC article. Clinical Trial. - Efficacy of Combining Varenicline and Naltrexone for Smoking Cessation and Drinking Reduction: A Randomized Clinical Trial.
Ray LA, Green R, Enders C, Leventhal AM, Grodin EN, Li G, Lim A, Hartwell E, Venegas A, Meredith L, Nieto SJ, Shoptaw S, Ho D, Miotto K. Ray LA, et al. Am J Psychiatry. 2021 Sep 1;178(9):818-828. doi: 10.1176/appi.ajp.2020.20070993. Epub 2021 Jun 3. Am J Psychiatry. 2021. PMID: 34080890 Free PMC article. Clinical Trial. - Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes.
Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Anton RF, et al. Alcohol Clin Exp Res. 2012 Nov;36(11):2000-7. doi: 10.1111/j.1530-0277.2012.01807.x. Epub 2012 May 2. Alcohol Clin Exp Res. 2012. PMID: 22551036 Free PMC article. Clinical Trial. - The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking.
Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. Pettinati HM, et al. J Clin Psychopharmacol. 2006 Dec;26(6):610-25. doi: 10.1097/01.jcp.0000245566.52401.20. J Clin Psychopharmacol. 2006. PMID: 17110818 Review. - The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis.
Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. Donoghue K, et al. Addiction. 2015 Jun;110(6):920-30. doi: 10.1111/add.12875. Epub 2015 Mar 17. Addiction. 2015. PMID: 25664494 Review.
Cited by
- Advances in the science and treatment of alcohol use disorder.
Witkiewitz K, Litten RZ, Leggio L. Witkiewitz K, et al. Sci Adv. 2019 Sep 25;5(9):eaax4043. doi: 10.1126/sciadv.aax4043. eCollection 2019 Sep. Sci Adv. 2019. PMID: 31579824 Free PMC article. Review. - Inclusion of Cannabis Users in Alcohol Research Samples: Screening In, Screening Out, and Implications.
Venegas A, Meredith LR, Cooper ZD, Towns B, Ray LA. Venegas A, et al. Alcohol Alcohol. 2020 Jun 25;55(4):416-423. doi: 10.1093/alcalc/agaa023. Alcohol Alcohol. 2020. PMID: 32328657 Free PMC article. - Genetic deletion or pharmacological blockade of nociceptin/orphanin FQ receptors in the ventral tegmental area attenuates nicotine-motivated behaviour.
Domi A, Lunerti V, Petrella M, Domi E, Borruto AM, Ubaldi M, Weiss F, Ciccocioppo R. Domi A, et al. Br J Pharmacol. 2022 Jun;179(11):2647-2658. doi: 10.1111/bph.15762. Epub 2022 Feb 10. Br J Pharmacol. 2022. PMID: 34854073 Free PMC article. - Evidence of subgroup differences in meta-analyses evaluating medications for alcohol use disorder: An umbrella review.
Wallach JD, Glick L, Gueorguieva R, O'Malley SS. Wallach JD, et al. Alcohol Clin Exp Res (Hoboken). 2024 Jan;48(1):5-15. doi: 10.1111/acer.15229. Epub 2023 Dec 15. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 38102794 Free PMC article. Review. - Smoking As an Outcome Moderator In the Treatment of Alcohol Use Disorders.
van Amsterdam J, van den Brink W. van Amsterdam J, et al. Alcohol Alcohol. 2022 Nov 11;57(6):664-673. doi: 10.1093/alcalc/agac027. Alcohol Alcohol. 2022. PMID: 35589093 Free PMC article. Review.
References
- ANTON RF, LIEBER C, TABAKOFF B, CDTECT STUDY GROUP Carbohydrate deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcoholism: Clinical and Experimental Research. 2002;26:1215–1222. - PubMed
- ANTON RF, O'MALLEY SS, CIRAULO DA, CISLER RA, COUPER D, DONOVAN DM, GASTFRIEND DR, HOSKING JD, JOHNSON BA, LOCASTRO JS, LONGABAUGH R, MASON BJ, MATTSON ME, MILLER WR, PETTINATI HM, RANDALL CL, SWIFT R, WEISS RD, WILLIAMS LD, ZWEBEN A, GROUP, C. S. R. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–17. - PubMed
- ANTON RF, OROSZI G, O'MALLEY SS, COUPER DJ, SWIFT R, PETTINATI HM, GOLDMAN D. An evaluation of µ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the combined pharmacotherapies and behavioral interventions for alcohol dependence (COMBINE) study. Archives of General Psychiatry. 2008;65:135–144. - PMC - PubMed
- ANTON RF, YOUNGBLOOD M. Factors affecting %CDT status at entry into a multisite clinical treatment trial: experience from the COMBINE Study. Alcohol Clin Exp Res. 2006;30:1878–83. - PubMed
- BENJAMIN D, GRANT ER, POHORECKY LA. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res. 1993;621:137–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K05 AA017435/AA/NIAAA NIH HHS/United States
- P50 AA010761/AA/NIAAA NIH HHS/United States
- R00 AA021419/AA/NIAAA NIH HHS/United States
- R01 AA017633/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous