Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial - PubMed (original) (raw)
Clinical Trial
. 2018 Mar;19(3):405-415.
doi: 10.1016/S1470-2045(18)30081-0. Epub 2018 Feb 10.
Elizabeth R Plimack 2, Igor Puzanov 3, Mayer N Fishman 4, David F McDermott 5, Daniel C Cho 6, Ulka Vaishampayan 7, Saby George 8, Thomas E Olencki 9, Jamal C Tarazi 10, Brad Rosbrook 10, Kathrine C Fernandez 11, Mariajose Lechuga 12, Toni K Choueiri 13
Affiliations
- PMID: 29439857
- PMCID: PMC6860026
- DOI: 10.1016/S1470-2045(18)30081-0
Clinical Trial
Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial
Michael B Atkins et al. Lancet Oncol. 2018 Mar.
Abstract
Background: Previous studies combining PD-1 checkpoint inhibitors with tyrosine kinase inhibitors of the VEGF pathway have been characterised by excess toxicity, precluding further development. We hypothesised that axitinib, a more selective VEGF inhibitor than others previously tested, could be combined safely with pembrolizumab (anti-PD-1) and yield antitumour activity in patients with treatment-naive advanced renal cell carcinoma.
Methods: In this ongoing, open-label, phase 1b study, which was done at ten centres in the USA, we enrolled patients aged 18 years or older who had advanced renal cell carcinoma (predominantly clear cell subtype) with their primary tumour resected, and at least one measureable lesion, Eastern Cooperative Oncology Group performance status 0-1, controlled hypertension, and no previous systemic therapy for renal cell carcinoma. Eligible patients received axitinib plus pembrolizumab in a dose-finding phase to estimate the maximum tolerated dose, and additional patients were enrolled into a dose-expansion phase to further establish safety and determine preliminary efficacy. Axitinib 5 mg was administered orally twice per day with pembrolizumab 2 mg/kg given intravenously every 3 weeks. We assessed safety in all patients who received at least one dose of axitinib or pembrolizumab; antitumour activity was assessed in all patients who received study treatment and had an adequate baseline tumour assessment. The primary endpoint was investigator-assessed dose-limiting toxicity during the first two cycles (6 weeks) to estimate the maximum tolerated dose and recommended phase 2 dose. This study is registered with ClinicalTrials.gov, number NCT02133742.
Findings: Between Sept 23, 2014, and March 25, 2015, we enrolled 11 patients with previously untreated advanced renal cell carcinoma to the dose-finding phase and between June 3, 2015, and Oct 13, 2015, we enrolled 41 patients to the dose-expansion phase. All 52 patients were analysed together. No unexpected toxicities were observed. Three dose-limiting toxicities were reported in the 11 patients treated during the 6-week observation period (dose-finding phase): one patient had a transient ischaemic attack and two patients were only able to complete less than 75% of the planned axitinib dose because of treatment-related toxicity. At the data cutoff date (March 31, 2017), 25 (48%) patients were still receiving study treatment. Grade 3 or worse treatment-related adverse events occurred in 34 (65%) patients; the most common included hypertension (n=12 [23%]), diarrhoea (n=5 [10%]), fatigue (n=5 [10%]), and increased alanine aminotransferase concentration (n=4 [8%]). The most common potentially immune-related adverse events (probably related to pembrolizumab) included diarrhoea (n=15 [29%]), increased alanine aminotransferase concentration (n=9 [17%]) or aspartate aminotransferase concentration (n=7 [13%]), hypothyroidism (n=7 [13%]), and fatigue (n=6 [12%]). 28 (54%) patients had treatment-related serious adverse events. At data cutoff, 38 (73%; 95% CI 59·0-84·4) patients achieved an objective response (complete or partial response).
Interpretation: The treatment combination of axitinib plus pembrolizumab is tolerable and shows promising antitumour activity in patients with treatment-naive advanced renal cell carcinoma. Whether or not the combination works better than a sequence of VEGF pathway inhibition followed by an anti-PD-1 therapy awaits the completion of a phase 3 trial comparing axitinib plus pembrolizumab with sunitinib monotherapy (NCT02853331).
Funding: Pfizer Inc.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
JCT, BR, ML, and KCF are employees of and own stock in Pfizer.
MBA declares receiving fees for consulting from Bristol-Myers Squibb (BMS), Pfizer, Novartis, Genentech-Roche, Merck, and Eisai. ERP declares receiving fees for consulting from AstraZeneca, BMS, Clovis, Eli Lilly and Company, Exelexis, Genentech, Horizon Pharma, Inovio, Novartis, Pfizer, and Roche, and grant support to her institution has been received from AstraZeneca, BMS, Merck, Peloton, Pfizer, GlaxoSmithKline (GSK), Dendreon, Aveo, Acceleron, and Eli Lilly Inc. MNF has received research funding from BMS, Exelixis, Eisai, Genentech, Acceleron, Merck, Prometheus, Nektar, Alkermes, and Pfizer, and has served on speakers bureaus for Exelixis. DFM declares receiving fees for consulting from BMS, Pfizer, Novartis, Genentech-Roche, Merck, Eisai, Array BioPharm, Prometheus, and Exelixis. SG declares receiving fees for consulting and serving on advisory boards from Pfizer, Exelixis, BMS, Novartis, Bayer, Janssen, Corvus, and AstraZeneca, and institutional grant support from BMS, Novartis, Bayer, Pfizer, Merck, and Agensys. TKC declares receiving fees for consulting and for serving on advisory boards from GSK, Novartis, Pfizer, Merck, AstraZeneca, Bayer, Genentech, Exelixis, Eisai, Cerulean, Foundation Medicine Inc, Corvus, and Prometheus, and grant support through his institution from BMS, GSK, Novartis, Exelixis, Pfizer, Merck, Roche, AstraZeneca, TRACON Pharmaceuticals, and Peloton. DCC declares receiving fees for consulting from Pfizer, Genentech, Prometheus, BMS, and Exelixis. UV declares research support from Astellas, Novartis, and Exelixis and consulting fees from Pfizer, Bayer, and BMS. All other authors declare no competing interests.
Figures
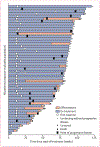
Figure 1:. Tumour swimmer plot for the response-evaluable population (n=52)
*Patient discontinued but had no off-treatment scan.
Figure 2:. Percentage change in (A) tumour burden by best response and (B) lesion diameters over time
(A) Percentage change in tumour burden by best response. The horizontal line at −30% change in tumour size from baseline represents the RECIST version 1.1 cutoff to define partial response or complete response. One patient with stable disease had no change and so was not visible. Another patient, labelled indeterminate, had no follow-up and was excluded from the plot. The patient with progressive disease as best response and 100% tumour shrinkage had an increased size of one lesion that indicated progressive disease on his second scan. This patient remained on treatment and on day 417 met partial response criteria; on day 669 the patient had 100% tumour shrinkage and a complete response. (B) Percentage change in lesion diameters over time. Two patients who had a complete response but do not appear on the chart achieved complete response after months 21 and 22. SLD of all target lesions was used for tumour size calculation at baseline and at all visits. Maximal change in lesion diameters as percentage change was plotted for each patient. SLD=sum of the lesion diameter. *Stable disease or partial response not confirmed, or no follow-up scans available.
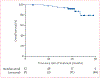
Figure 3:. Progression-free survival
Points on the curve represent censored patients.
Figure 4:. Overall survival
Points on the curve represent censored patients.
Comment in
- Combination therapies for patients with metastatic renal cell carcinoma.
Procopio G, Ratta R, de Braud F, Verzoni E. Procopio G, et al. Lancet Oncol. 2018 Mar;19(3):281-283. doi: 10.1016/S1470-2045(18)30092-5. Epub 2018 Feb 10. Lancet Oncol. 2018. PMID: 29439858 No abstract available. - Axitinib plus Pembrolizumab Is Effective in Renal Cell Carcinoma.
[No authors listed] [No authors listed] Cancer Discov. 2018 Apr;8(4):OF6. doi: 10.1158/2159-8290.CD-RW2018-032. Epub 2018 Feb 23. Cancer Discov. 2018. PMID: 29475884 - Combination VEGFR/immune checkpoint inhibitor therapy: a promising new treatment for renal cell carcinoma.
Lee CH, Motzer R. Lee CH, et al. Br J Cancer. 2018 Oct;119(8):911-912. doi: 10.1038/s41416-018-0175-x. Epub 2018 Oct 17. Br J Cancer. 2018. PMID: 30327569 Free PMC article. No abstract available.
Similar articles
- Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial.
Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, Powles T, McDermott D, Robbins PB, Chism DD, Cho D, Atkins MB, Gordon MS, Gupta S, Uemura H, Tomita Y, Compagnoni A, Fowst C, di Pietro A, Rini BI. Choueiri TK, et al. Lancet Oncol. 2018 Apr;19(4):451-460. doi: 10.1016/S1470-2045(18)30107-4. Epub 2018 Mar 9. Lancet Oncol. 2018. PMID: 29530667 Clinical Trial. - Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial.
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, Azevedo SJ, Borchiellini D, McDermott RS, Bedke J, Tamada S, Yin L, Chen M, Molife LR, Atkins MB, Rini BI. Powles T, et al. Lancet Oncol. 2020 Dec;21(12):1563-1573. doi: 10.1016/S1470-2045(20)30436-8. Epub 2020 Oct 23. Lancet Oncol. 2020. PMID: 33284113 Clinical Trial. - Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial.
Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, Bendell J, Penel N, Krebs MG, Martin-Liberal J, Isambert N, Soriano A, Wermke M, Cultrera J, Gao L, Widau RC, Mi G, Jin J, Ferry D, Fuchs CS, Petrylak DP, Chau I. Herbst RS, et al. Lancet Oncol. 2019 Aug;20(8):1109-1123. doi: 10.1016/S1470-2045(19)30458-9. Epub 2019 Jul 10. Lancet Oncol. 2019. PMID: 31301962 Clinical Trial. - Pembrolizumab plus axitinib for the treatment of advanced renal cell carcinoma.
Spisarová M, Melichar B, Vitásková D, Študentová H. Spisarová M, et al. Expert Rev Anticancer Ther. 2021 Jul;21(7):693-703. doi: 10.1080/14737140.2021.1903321. Epub 2021 Apr 2. Expert Rev Anticancer Ther. 2021. PMID: 33794744 Review. - Pembrolizumab in Combination with Axitinib as First-Line Treatment for Patients with Renal Cell Carcinoma (RCC): Evidence to Date.
Chau V, Bilusic M. Chau V, et al. Cancer Manag Res. 2020 Aug 17;12:7321-7330. doi: 10.2147/CMAR.S216605. eCollection 2020. Cancer Manag Res. 2020. PMID: 32884346 Free PMC article. Review.
Cited by
- Interaction between Immunotherapy and Antiangiogenic Therapy for Cancer.
Furukawa K, Nagano T, Tachihara M, Yamamoto M, Nishimura Y. Furukawa K, et al. Molecules. 2020 Aug 26;25(17):3900. doi: 10.3390/molecules25173900. Molecules. 2020. PMID: 32859106 Free PMC article. Review. - First-Line Systemic Therapy for Metastatic Clear-Cell Renal Cell Carcinoma: Critical Appraisal of Emerging Options.
Loo V, Salgia M, Bergerot P, Philip EJ, Pal SK. Loo V, et al. Target Oncol. 2019 Dec;14(6):639-645. doi: 10.1007/s11523-019-00676-y. Target Oncol. 2019. PMID: 31595385 Review. - Preclinical rationale and clinical efficacy of antiangiogenic therapy and immune checkpoint blockade combination therapy in urogenital tumors.
Zhu N, Weng S, Wang J, Chen J, Yu L, Fang X, Yuan Y. Zhu N, et al. J Cancer Res Clin Oncol. 2019 Dec;145(12):3021-3036. doi: 10.1007/s00432-019-03044-5. Epub 2019 Oct 15. J Cancer Res Clin Oncol. 2019. PMID: 31617075 Review. - Combination of Anti-Angiogenics and Checkpoint Inhibitors for Renal Cell Carcinoma: Is the Whole Greater Than the Sum of Its Parts?
Jonasch E, Atkins MB, Chowdhury S, Mainwaring P. Jonasch E, et al. Cancers (Basel). 2022 Jan 27;14(3):644. doi: 10.3390/cancers14030644. Cancers (Basel). 2022. PMID: 35158916 Free PMC article. Review. - VEGF in Signaling and Disease: Beyond Discovery and Development.
Apte RS, Chen DS, Ferrara N. Apte RS, et al. Cell. 2019 Mar 7;176(6):1248-1264. doi: 10.1016/j.cell.2019.01.021. Cell. 2019. PMID: 30849371 Free PMC article. Review.
References
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–77. - PubMed
- Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66: 3381–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical