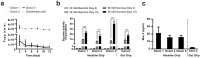Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids - PubMed (original) (raw)
doi: 10.1038/s41598-018-21201-7.
Alessio Tovaglieri 1 3, Alexandra Sontheimer-Phelps 1 4, Sasan Jalili-Firoozinezhad 1 5, Amir Bein 1, Angeliki Chalkiadaki 1, William Scholl 1, Cheng Zhang 6, Hannah Rickner 7, Camilla A Richmond 8 9, Hu Li 6, David T Breault 10 11 12, Donald E Ingber 13 14 15
Affiliations
- PMID: 29440725
- PMCID: PMC5811607
- DOI: 10.1038/s41598-018-21201-7
Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids
Magdalena Kasendra et al. Sci Rep. 2018.
Abstract
Here we describe a method for fabricating a primary human Small Intestine-on-a-Chip (Intestine Chip) containing epithelial cells isolated from healthy regions of intestinal biopsies. The primary epithelial cells are expanded as 3D organoids, dissociated, and cultured on a porous membrane within a microfluidic device with human intestinal microvascular endothelium cultured in a parallel microchannel under flow and cyclic deformation. In the Intestine Chip, the epithelium forms villi-like projections lined by polarized epithelial cells that undergo multi-lineage differentiation similar to that of intestinal organoids, however, these cells expose their apical surfaces to an open lumen and interface with endothelium. Transcriptomic analysis also indicates that the Intestine Chip more closely mimics whole human duodenum in vivo when compared to the duodenal organoids used to create the chips. Because fluids flowing through the lumen of the Intestine Chip can be collected continuously, sequential analysis of fluid samples can be used to quantify nutrient digestion, mucus secretion and establishment of intestinal barrier function over a period of multiple days in vitro. The Intestine Chip therefore may be useful as a research tool for applications where normal intestinal function is crucial, including studies of metabolism, nutrition, infection, and drug pharmacokinetics, as well as personalized medicine.
Conflict of interest statement
The authors declare potential competing financial interests as D.E.I is a founder, holds equity and chairs the scientific advisory board of Emulate Inc.
Figures
Figure 1
Fabrication of the primary human Intestine Chip. (a) A schematic cross-sectional view (top) and a phase contrast micrograph of the chip viewed from above (bottom) showing the upper (epithelial; blue) and lower (microvascular; pink) cell culture microchannels separated by a porous, ECM-coated, PDMS membrane sandwiched in-between. The membrane is elastic and can be extended and retracted by the application of cyclic vacuum to the hollow side chambers. This actuation causes outward deflection of the vertical side walls and lateral extension of the attached horizontal, porous elastic membrane, which induces mechanical deformation of the adherent tissue layers cultured in the central channels. (b) Schematic representation of the step-by-step procedure involved in the establishment of microfluidic co-cultures of primary human intestinal epithelium and intestinal microvascular endothelium in the Intestine Chip.
Figure 2
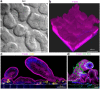
Morphological analysis of Organ Chips lined by primary duodenal organoid-derived epithelial cells in the absence of endothelial cells. (a) Microscopic views showing the finger-like potrusions of the primary intestinal epithelium cultured on-chip for 12 days under continuous flow (60 µl hr−1), when viewed from above by DIC imaging. (b) Representative 3D reconstruction of confocal immunofluorescence micrographs of organoid-derived intestinal epithelium grown on-chip (magenta, F-actin; blue, DAPI-stained nuclei). (c) Representative vertical cross sectional view of confocal microscopic images showing intestinal epithelium immunostained for F-actin (magenta) and Ki67 (yellow). (d) Representative vertical cross sectional, confocal, micrographic views through the intestinal epithelium-membrane interface of the intestinal epithelium grown on-chip when immunostained for F-actin (magenta) and Muc5AC (green), and nuclei with DAPI (blue) (in c and d, white dashed lines indicate upper surfaces of the porous matrix-coated membrane).
Figure 3
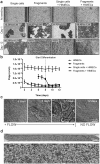
Establishment of the primary human intestinal epithelium in the Organ Chip in the presence or absence intestine-specific microvascular endothelium. (a) Comparison of the efficiency of intestinal epithelial cell monolayer formation and development of villi-like structures between Organ Chip devices seeded with single intestinal epithelial cells or organoid fragments in the presence or absence of human intestinal microvascular endothelial cells (HIMECs). All chips were maintained for 1 or 12 days under continuous apical fluid flow of expansion (day 1–8) and differentiation media (day 9–12) and basal perfusion of EGM2-MV medium. White areas delineate empty spaces where initially plated cells detached from the membrane when flow was initiated; dark areas represent patches of attached cells. Graph shows the percentage of the area covered by epithelium at days 1 and 12 of culture, as assessed across 3 different field of views/chip with at least 3 chip replicates per condition, and expressed as mean ±SEM from 3 independent experiments. Note that when EGM2-MV medium was perfused basally, epithelium formed intestinal villi-like structures only when co-cultured with HIMECs seeded on the opposite side of the porous membrane. (b) Graph showing maturation of the intestinal barrier function on chips cultured under the same conditions measured by quantifying permeability of fluorescent Lucifer Yellow. Papp values are presented as mean ± SEM from 3 independent experiments; only Papp values for the cultures that reached 100% of confluency are plotted here. Note that the epithelium develops a higher barrier resistance to Lucifer Yellow more quickly when HIMECs are present. (c) Representative differential interference contrast images of the primary intestinal epithelium cultured on chip with HIMECs for 4, 6 or 12 days under continuous flow compared to 12 days in the absence of flow (note that formation of intestinal vill-like structures occurred only in the presence of flow). (d) Phase contrast microscopy views of the entire length of the epithelial microchannel (top) and a higher magnification of the area highlighted in the white rectangle (bottom) showing the human primary intestinal epithelium co-cultured with intestinal microvascular endothelial cells in the Organ Chip under peristalsis-like motions and flow for 12 days (culture medium was switched from EM to DM on day 8). Note the presence of villi-like structures across the entire length of the channel.
Figure 4
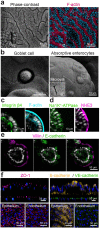
Morphological analysis of the primary human Intestine Chip. (a) Representative immunofluorescence microscopic views from above of the intestinal epithelium grown on-chip for 12 days under fluid flow and cyclic strain showing the presence of a continuous brush border along the apical membranes of epithelial villi-like protrusions, which are juxtaposed in close proximity, as visualized by labeling of the brush border for F-actin (magenta) and for nuclei with DAPI (blue). (b) High magnification SEMs of the apical surface of the epithelium cultured on-chip showing a
g
oblet cell (left) and absorptive enterocytes (right) with apical microvilli. (c–e) Immunofluorescence micrographs of the Intestine Chip showing polarized distribution of apical (F-actin,NHE3, villin) as well as basal (integrin β4) and basolateral (Na+/K+-ATPase,E-cadherin) proteins in the cross-sectional views of single villus-like structure all counterstained with DAPI (grey).(c) Integrin β4 (green) and F-actin (cyan). (d) Na+/K+-ATPase (green) and NHE3 (magenta), (e) Villin (magenta) and E-cadherin (green) (f) Confocal immunofluorescence micrographs showing the presence of apical intact tight junctions in the intestinal epithelium and underlying endothelium labeled with ZO-1 (magenta) as well as adherens junctions labeled for E-cadherin (yellow) and VE-cadherin (green); blue indicates DAPI-stained nuclei.
Figure 5
The primary human Intestine Chip exhibits multi-lineage differentiation. (a) Graph showing the fold change in the levels of transcripts from epithelial cells cultured in the Intestine Chip and assessed at day 12 relative to the transcript levels in the undifferentiated state assessed at the day 8 (the medium perfused through the apical channel was switched from EM to DM on day 8). Genes analyzed include sucrose-isomaltase (SI) and alkaline phosphatase (ALPI) that are specific for absorptive enterocytes; mucin 2 (MUC2) and mucin 5AC (MUC5AC) for goblet cells; chromogranin A (CHGA) and synaptophysin (SYP) for enteroendocrine cells; lysozyme (LYZ) for Paneth cells; and leucine-rich-repeat-containing G-protein-coupled receptor 5 (LGR5) and polycomb complex protein BMI-1 (BMI1) for stem cells. Values are presented as mean ±SEM from 3 independent experiments involving Intestine Chips generated from organoids isolated from 3 different donors (ns, not significant; *p ≤ 0.05; ***p ≤ 0.001 by Student’s t-test). (b) A schematic cross-sectional representation of the 3D intestinal epithelial tissue architecture developed on chip (top) and confocal immunofluorescence micrographs (bottom) showing vertical cross-sections of the differentiated epithelium in Intestine Chip stained for lysozyme (Lyz, green), mucin 2 (Muc2, magenta), chromogranin A (ChgA, yellow) and villin (green). Cell nuclei were counterstained with DAPI (blue).
Figure 6
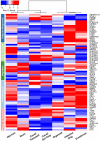
The Intestine Chip resembles human native duodenum. A curated heatmap was generated for genes selected through z-score comparison and template matching method showing genes with similar expression profiles in the mechanically active Intestine Chip (with fluid flow at 60 μL h−1 and cyclic stretching at 10%, 0.2 Hz; Intestine Chip) and in vivo duodenum in comparison to in vivo jejunum and ileum, as well as 3D organoids and Caco-2 based Gut Chip (Caco-2 Gut Chip) and static Caco-2 Transwell models. 3D organoids and Intestine Chip were established from the same duodenal tissue biopsies derived from 3 healthy donors and differentiated for 4 days in DM medium before assessment. Gene expression data for normal human small intestine (duodenum, jejunum, and ileum), Caco-2 Gut Chip, and Caco-2 Transwell were obtained from Gene Expression Omnibus (GEO) database,. Selected genes shown belong to the following GO terms: Defense Response (GO:0006952), Drug Transport (GO:0015893), Digestive System Process (GO:0022600), Regulation of Epithelial Cell Proliferation (GO:0050678), and Response to Nutrients (GO:0007584). See Supplementary Table S1 for full names of all genes and GO categories.
Figure 7
Modeling normal intestinal physiology in the Intestine Chip. (a) Comparison of barrier function measured in the intact Intestine Chips lined with epithelium derived from biopsies from 3 different donors and primary HIMECs versus chips lined by endothelium alone by measuring paracellular passage of 450 Da Lucifer Yellow over 2 to 12 days of culture. Data are presented as mean ± SEM of 3 independent experiments involving Intestine Chips generated from organoids derived from three different donors (3 chips/donor). (b) Comparison of intestinal epithelial cell sucrase-isomaltase activity in the Intestine Chip and Caco-2 Gut Chip showing that specific enzyme activity increases in both from 4 to 12 days of culture as the cells differentiate into mature absorptive enterocytes. The increased concentration of glucose in the chip effluents results from hydrolysis of 30 mM sucrose which results in formation of glucose and fructose; note that this does not occur when 30 mM mannitol is used instead of sucrose. Data are presented as means ± SEM from 3 independent experiments, each involving 3 different Caco2 Chips and 3 individual chips/organoid donor (***p < 0.001 by ANOVA). (c) Comparison of mucin 2 (Muc2) levels in the apical secretions collected in the effluent from the epithelial channel of the Intestine Chip generated from organoids derived from 3 different donors versus that measured in effluents of a human Caco-2 cell line-based Gut Chip at day 12. Data represent means ± SEM from 3 independent experiments involving 3 different Caco-2 Chips or Chips from 3 different donors (3 chips/donor).
Similar articles
- Combining Human Organoids and Organ-on-a-Chip Technology to Model Intestinal Region-Specific Functionality.
Kulkarni G, Apostolou A, Ewart L, Lucchesi C, Kasendra M. Kulkarni G, et al. J Vis Exp. 2022 May 5;(183). doi: 10.3791/63724. J Vis Exp. 2022. PMID: 35604153 - A Novel Microphysiological Colon Platform to Decipher Mechanisms Driving Human Intestinal Permeability.
Apostolou A, Panchakshari RA, Banerjee A, Manatakis DV, Paraskevopoulou MD, Luc R, Abu-Ali G, Dimitriou A, Lucchesi C, Kulkarni G, Maulana TI, Kasendra M, Kerns JS, Bleck B, Ewart L, Manolakos ES, Hamilton GA, Giallourakis C, Karalis K. Apostolou A, et al. Cell Mol Gastroenterol Hepatol. 2021;12(5):1719-1741. doi: 10.1016/j.jcmgh.2021.07.004. Epub 2021 Jul 17. Cell Mol Gastroenterol Hepatol. 2021. PMID: 34284165 Free PMC article. - Intestinal Models for Personalized Medicine: from Conventional Models to Microfluidic Primary Intestine-on-a-chip.
Li XG, Chen MX, Zhao SQ, Wang XQ. Li XG, et al. Stem Cell Rev Rep. 2022 Aug;18(6):2137-2151. doi: 10.1007/s12015-021-10205-y. Epub 2021 Jun 28. Stem Cell Rev Rep. 2022. PMID: 34181185 Free PMC article. Review. - Intestinal organoids in farm animals.
Beaumont M, Blanc F, Cherbuy C, Egidy G, Giuffra E, Lacroix-Lamandé S, Wiedemann A. Beaumont M, et al. Vet Res. 2021 Feb 25;52(1):33. doi: 10.1186/s13567-021-00909-x. Vet Res. 2021. PMID: 33632315 Free PMC article. Review. - Organ-on-Chip Approaches for Intestinal 3D In Vitro Modeling.
Pimenta J, Ribeiro R, Almeida R, Costa PF, da Silva MA, Pereira B. Pimenta J, et al. Cell Mol Gastroenterol Hepatol. 2022;13(2):351-367. doi: 10.1016/j.jcmgh.2021.08.015. Epub 2021 Aug 25. Cell Mol Gastroenterol Hepatol. 2022. PMID: 34454168 Free PMC article. Review.
Cited by
- A Microphysiological System with an Anaerobic Air-Liquid Interface and Functional Mucus Layer for Coculture of Intestinal Bacteria and Primary Human Colonic Epithelium.
Kim R, Allbritton NL. Kim R, et al. Adv Mater Interfaces. 2024 Sep 3;11(25):2400093. doi: 10.1002/admi.202400093. Epub 2024 Jun 19. Adv Mater Interfaces. 2024. PMID: 39386255 - Human Mesofluidic Intestinal Model for Studying Transport of Drug Carriers and Bacteria Through a Live Mucosal Barrier.
Wang CM, Oberoi HS, Law D, Li Y, Kassis T, Griffith LG, Breault DT, Carrier RL. Wang CM, et al. bioRxiv [Preprint]. 2024 Sep 22:2024.09.18.613692. doi: 10.1101/2024.09.18.613692. bioRxiv. 2024. PMID: 39345622 Free PMC article. Preprint. - A comprehensive overview of advanced dynamic in vitro intestinal and hepatic cell culture models.
Leal F, Zeiringer S, Jeitler R, Costa PF, Roblegg E. Leal F, et al. Tissue Barriers. 2024 Jan 2;12(1):2163820. doi: 10.1080/21688370.2022.2163820. Epub 2023 Jan 21. Tissue Barriers. 2024. PMID: 36680530 Free PMC article. Review. - 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert.
Shin W, Kim HJ. Shin W, et al. Nat Protoc. 2022 Mar;17(3):910-939. doi: 10.1038/s41596-021-00674-3. Epub 2022 Feb 2. Nat Protoc. 2022. PMID: 35110737 Free PMC article. Review. - Organoid technologies for the study of intestinal microbiota-host interactions.
Bozzetti V, Senger S. Bozzetti V, et al. Trends Mol Med. 2022 Apr;28(4):290-303. doi: 10.1016/j.molmed.2022.02.001. Epub 2022 Feb 26. Trends Mol Med. 2022. PMID: 35232671 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- R01 DK084056/DK/NIDDK NIH HHS/United States
- HHSF223201310079C/FD/FDA HHS/United States
- T32 DK007477/DK/NIDDK NIH HHS/United States
- F32 DK091995/DK/NIDDK NIH HHS/United States
- K08 DK106562/DK/NIDDK NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases