Humidity-dependent wound sealing in succulent leaves of Delosperma cooperi - An adaptation to seasonal drought stress - PubMed (original) (raw)
Humidity-dependent wound sealing in succulent leaves of Delosperma cooperi - An adaptation to seasonal drought stress
Olga Speck et al. Beilstein J Nanotechnol. 2018.
Abstract
During evolution, plants evolved various reactions to wounding. Fast wound sealing and subsequent healing represent a selective advantage of particular importance for plants growing in arid habitats. An effective self-sealing function by internal deformation has been found in the succulent leaves of Delosperma cooperi. After a transversal incision, the entire leaf bends until the wound is closed. Our results indicate that the underlying sealing principle is a combination of hydraulic shrinking and swelling as the main driving forces and growth-induced mechanical pre-stresses in the tissues. Hydraulic effects were measured in terms of the relative bending angle over 55 minutes under various humidity conditions. The higher the relative air humidity, the lower the bending angle. Negative bending angles were found when a droplet of liquid water was applied to the wound. The statistical analysis revealed highly significant differences of the single main effects such as "humidity conditions in the wound region" and "time after wounding" and their interaction effect. The centripetal arrangement of five tissue layers with various thicknesses and significantly different mechanical properties might play an additional role with regard to mechanically driven effects. Injury disturbs the mechanical equilibrium, with pre-stresses leading to internal deformation until a new equilibrium is reached. In the context of self-sealing by internal deformation, the highly flexible wide-band tracheids, which form a net of vascular bundles, are regarded as paedomorphic tracheids, which are specialised to prevent cell collapse under drought stress and allow for building growth-induced mechanical pre-stresses.
Keywords: hydraulics; mechanical pre-stress; paedomorphosis; self-sealing; wide band tracheids.
Figures
Figure 1
Delosperma cooperi. (a) Flowering plants cultivated outdoors in the Botanic Garden Freiburg, Germany. (b) Permanent kink (arrow) after wounding and finalised self-repair of a succulent leaf. Scale bars equal 5 mm.
Figure 2
Anatomy of adult leaves of Delosperma cooperi. (a) Cross-section of the entire leaf (stained with toluidine blue) comprising five tissue layers: epidermis (ep) with window cells (wc), chlorenchyma (ch), net of peripheral vascular bundles (nvb), parenchyma (pa) and central vascular bundle (cvb). (b, c) Cross-sections of vascular tissues with xylem (xy) and phloem (ph), (b) central vascular bundle, (c) peripheral vascular bundle with wide-band tracheids showing pronounced cell wall thickenings.
Figure 3
Self-sealing efficiency of Delosperma cooperi leaves. Diagrams show the relative bending angle as a function of “humidity conditions in the wound region” (r.h. = relative air humidity and water droplet) and “time after wounding”. (a–f) Comparative box and whisker diagrams display the relative bending angles γn dependent on “water” in terms of relative air humidity (r.h.) at 24, 49 and 100% and after a water droplet was applied to a transversal wound after (a) 1 min, (b) 5 min, (c) 10 min, (d) 20 min, (e) 40 min and (f) 55 min. (g) For better traceability the significant interaction effect of “humidity conditions in the wound region” and “time after wounding” is presented as the median of measured data (note that the statistical analysis was carried out on rank-transformed data showing the same results).
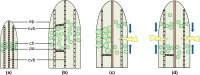
Figure 4
Schematic drawings of intact and damaged leaves. (a) Juvenile leaf, (b) adult leaf, (c) transversal or longitudinal cut, (d) ring incision. Illustration of self-sealing (c,d) represents low relative humidity conditions with the arrows indicating the closure of the fissure due to leaf bending (c) or leaf contraction (d). (ep) epidermis with window cells, (ch) chlorenchyma, (nvb) net of peripheral vascular bundles, (pa) parenchyma, (cvb) central vascular bundle. Examples of parenchymatous cells are illustrated.
Figure 5
The evaluation of self-sealing. The tracking of three defined points at the leaf base (1), close to the injury (2) and at the leaf tip (3) during the bending of the injured leaf is the basis for calculating the angles α and β. Further calculations lead to the relative bending angle γ, which represents a quantitative measure for the self-sealing movement during a given time span.
Similar articles
- An analytic model of the self-sealing mechanism of the succulent plant Delosperma cooperi.
Konrad W, Flues F, Schmich F, Speck T, Speck O. Konrad W, et al. J Theor Biol. 2013 Nov 7;336:96-109. doi: 10.1016/j.jtbi.2013.07.013. Epub 2013 Jul 29. J Theor Biol. 2013. PMID: 23907028 - Comparative Analyses of the Self-Sealing Mechanisms in Leaves of Delosperma cooperi and Delosperma ecklonis (Aizoaceae).
Hesse L, Kampowski T, Leupold J, Caliaro S, Speck T, Speck O. Hesse L, et al. Int J Mol Sci. 2020 Aug 11;21(16):5768. doi: 10.3390/ijms21165768. Int J Mol Sci. 2020. PMID: 32796721 Free PMC article. - Finite element modelling of complex movements during self-sealing of ring incisions in leaves of Delosperma cooperi.
Klein H, Hesse L, Boljen M, Kampowski T, Butschek I, Speck T, Speck O. Klein H, et al. J Theor Biol. 2018 Dec 7;458:184-206. doi: 10.1016/j.jtbi.2018.08.023. Epub 2018 Aug 24. J Theor Biol. 2018. PMID: 30149008 - Cutinized and suberized barriers in leaves and roots: Similarities and differences.
Grünhofer P, Schreiber L. Grünhofer P, et al. J Plant Physiol. 2023 Mar;282:153921. doi: 10.1016/j.jplph.2023.153921. Epub 2023 Jan 11. J Plant Physiol. 2023. PMID: 36780757 Review. - Elastic and collapsible: current understanding of cell walls in succulent plants.
Fradera-Soler M, Grace OM, Jørgensen B, Mravec J. Fradera-Soler M, et al. J Exp Bot. 2022 Apr 18;73(8):2290-2307. doi: 10.1093/jxb/erac054. J Exp Bot. 2022. PMID: 35167681 Free PMC article. Review.
Cited by
- Assisted damage closure and healing in soft robots by shape memory alloy wires.
Kashef Tabrizian S, Terryn S, Cornellà AC, Brancart J, Legrand J, Van Assche G, Vanderborght B. Kashef Tabrizian S, et al. Sci Rep. 2023 May 31;13(1):8820. doi: 10.1038/s41598-023-35943-6. Sci Rep. 2023. PMID: 37258618 Free PMC article. - Longevity of System Functions in Biology and Biomimetics: A Matter of Robustness and Resilience.
Mylo MD, Speck O. Mylo MD, et al. Biomimetics (Basel). 2023 Apr 21;8(2):173. doi: 10.3390/biomimetics8020173. Biomimetics (Basel). 2023. PMID: 37092425 Free PMC article. Review. - Charting the twist-to-bend ratio of plant axes.
Wolff-Vorbeck S, Speck O, Langer M, Speck T, Dondl PW. Wolff-Vorbeck S, et al. J R Soc Interface. 2022 Jun;19(191):20220131. doi: 10.1098/rsif.2022.0131. Epub 2022 Jun 22. J R Soc Interface. 2022. PMID: 35730171 Free PMC article. - Acclimation to wind loads and/or contact stimuli? A biomechanical study of peltate leaves of Pilea peperomioides.
Langer M, Hegge E, Speck T, Speck O. Langer M, et al. J Exp Bot. 2022 Feb 24;73(4):1236-1252. doi: 10.1093/jxb/erab541. J Exp Bot. 2022. PMID: 34893822 Free PMC article. - Twist-to-Bend Ratios and Safety Factors of Petioles Having Various Geometries, Sizes and Shapes.
Langer M, Kelbel MC, Speck T, Müller C, Speck O. Langer M, et al. Front Plant Sci. 2021 Nov 11;12:765605. doi: 10.3389/fpls.2021.765605. eCollection 2021. Front Plant Sci. 2021. PMID: 34858462 Free PMC article.
References
- Harrington M J, Speck O, Speck T, Wagner S, Weinkamer R. Adv Polym Sci. 2016;273:307–344. doi: 10.1007/12_2015_334. - DOI
- Speck T, Bauer G, Flues F, Oelker K, Rampf M, Schüssele A C, v. Tapavicza M, Bertling J, Luchsinger R, Nellesen A, et al. Bio-inspired self-healing materials. In: Fratzl P, Dunlop J W C, Weinkamer R, editors. Materials design inspired by nature: function through inner architecture. Vol. 1. London, U.K.: The Royal Chemical Society; 2013. pp. 359–389.
- Speck T, Mülhaupt R, Speck O. Self-healing in plants as bio-inspiration for self-repairing polymers. In: Binder W H, editor. Self-Healing Polymers: From Principles to Applications. Weinheim, Germany: Wiley-VCH; 2013. pp. 69–97. - DOI
- Döhler D, Philipp M, Binder W. Principles of Self–Healing Polymers. In: Binder W H, editor. Self-Healing Polymers: From Principles to Applications. Weinheim, Germany: Wiley-VCH; 2013. pp. 5–60. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources