Endurance exercise training and high-fat diet differentially affect composition of diacylglycerol molecular species in rat skeletal muscle - PubMed (original) (raw)
Endurance exercise training and high-fat diet differentially affect composition of diacylglycerol molecular species in rat skeletal muscle
Noriaki Kawanishi et al. Am J Physiol Regul Integr Comp Physiol. 2018.
Abstract
Insulin resistance of peripheral muscle is implicated in the etiology of metabolic syndrome in obesity. Although accumulation of glycerolipids, such as triacylglycerol and diacylglycerol (DAG), in muscle contributes to insulin resistance in obese individuals, endurance-trained athletes also have higher glycerolipid levels but normal insulin sensitivity. We hypothesized that the difference in insulin sensitivity of skeletal muscle between athletes and obese individuals stems from changes in fatty acid composition of accumulated lipids. Here, we evaluated the effects of intense endurance exercise and high-fat diet (HFD) on the accumulation and composition of lipid molecular species in rat skeletal muscle using a lipidomic approach. Sprague-Dawley female rats were randomly assigned to three groups and received either normal diet (ND) in sedentary conditions, ND plus endurance exercise training, or HFD in sedentary conditions. Rats were fed ND or HFD between 4 and 12 wk of age. Rats in the exercise group ran on a treadmill for 120 min/day, 5 days/wk, for 8 wk. Soleus muscle lipidomic profiles were obtained using liquid chromatography/tandem mass spectrometry. Total DAG levels, particularly those of palmitoleate-containing species, were increased in muscle by exercise training. However, whereas the total DAG level in the muscle was also increased by HFD, the levels of DAG molecular species containing palmitoleate were decreased by HFD. The concentration of phosphatidylethanolamine molecular species containing palmitoleate was increased by exercise but decreased by HFD. Our results indicate that although DAG accumulation was similar levels in trained and sedentary obese rats, specific changes in molecular species containing palmitoleate were opposite.
Keywords: athlete’s paradox; diacylglycerol; skeletal muscle.
Figures
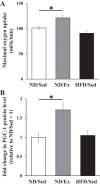
Fig. 1.
Effects of endurance exercise training and high-fat diet (HFD) on maximal oxygen uptake and peroxisome proliferator-activated receptor-γ coactivator (PGC-1) protein expression in skeletal muscle. A: maximal oxygen uptake of normal diet (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: relative protein levels of PGC-1 in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
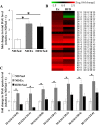
Fig. 2.
Effects of endurance exercise training and high-fat diet (HFD) on diacylglyceride (DAG) content in skeletal muscle. A: total DAG content in soleus muscle of normal (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: heat map showing the fold change of DAG molecular species content in soleus muscle of ND/Ex and HFD/Sed rats in comparison to the corresponding values in ND/Sed rats. C: relative levels of DAG molecular species in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as mean ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
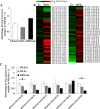
Fig. 3.
Effects of endurance exercise training and high-fat diet (HFD) on triacylglyceride (TAG) content in skeletal muscle. A: total TAG content in soleus muscle of normal diet ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: heat map showing the fold change of TAG molecular species content in soleus muscle of ND/Ex and HFD/Sed rats in comparison to the corresponding values in ND/Sed rats. C: relative levels of TAG molecular species in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
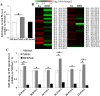
Fig. 4.
Effects of endurance exercise training and high-fat diet (HFD) on phosphatidylethanolamine (PE) content in skeletal muscle. A: total PE content in soleus muscle of normal diet (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: heat map showing the fold change of PE molecular species content in soleus muscle of ND/Ex and HFD/Sed rats in comparison to the corresponding values in ND/Sed rats. C: relative levels of PE molecular species in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
Fig. 5.
Effects of endurance exercise training and high-fat diet (HFD) on phosphatidylcholine (PC) content in skeletal muscle. A: total PC content in soleus muscle of normal diet (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: relative levels of PC molecular species in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
Fig. 6.
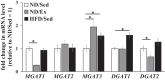
Effects of endurance exercise training and high-fat diet (HFD) on mRNA expression levels of monoacylglycerol acyltransferase (MGAT) and diacylglycerol acyltransferase (DGAT) genes in skeletal muscle. Relative mRNA levels of genes of MGAT and DGAT families in soleus muscle of normal diet (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
Fig. 7.
Effects of endurance exercise training and high-fat diet (HFD) on stearoyl-CoA desaturase 1 (SCD1) mRNA and protein expression in skeletal muscle. A: SCD1 mRNA levels in soleus muscle of normal diet (ND) plus sedentary (ND/Sed) rats, ND plus endurance exercise-training (ND/Ex) rats, and HFD plus sedentary (HFD/Sed) rats. B: SCD1 protein expression levels in soleus muscle of ND/Sed rats, ND/Ex rats, and HFD/Sed rats. Data are presented as means ± SE. *P < 0.05 compared with the ND/Sed rats by Dunnett’s multiple comparison test.
Similar articles
- Training status diverges muscle diacylglycerol accumulation during free fatty acid elevation.
Chow LS, Mashek DG, Austin E, Eberly LE, Persson XM, Mashek MT, Seaquist ER, Jensen MD. Chow LS, et al. Am J Physiol Endocrinol Metab. 2014 Jul 1;307(1):E124-31. doi: 10.1152/ajpendo.00166.2014. Epub 2014 May 20. Am J Physiol Endocrinol Metab. 2014. PMID: 24844260 Free PMC article. - Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet-induced hyperglycemia.
Smith AC, Mullen KL, Junkin KA, Nickerson J, Chabowski A, Bonen A, Dyck DJ. Smith AC, et al. Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E172-81. doi: 10.1152/ajpendo.00677.2006. Epub 2007 Mar 20. Am J Physiol Endocrinol Metab. 2007. PMID: 17374701 - Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle.
Steinberg GR, Smith AC, Wormald S, Malenfant P, Collier C, Dyck DJ. Steinberg GR, et al. Am J Physiol Endocrinol Metab. 2004 Jan;286(1):E57-63. doi: 10.1152/ajpendo.00302.2003. Am J Physiol Endocrinol Metab. 2004. PMID: 14662513 - Effect of acute endurance and resistance exercise on endocrine hormones directly related to lipolysis and skeletal muscle protein synthesis in adult individuals with obesity.
Hansen D, Meeusen R, Mullens A, Dendale P. Hansen D, et al. Sports Med. 2012 May 1;42(5):415-31. doi: 10.2165/11599590-000000000-00000. Sports Med. 2012. PMID: 22455310 Review.
Cited by
- Effects of Acute Exercise Combined With Calorie Restriction Initiated Late-in-Life on Insulin Signaling, Lipids, and Glucose Uptake in Skeletal Muscle From Old Rats.
Oki K, Arias EB, Kanzaki M, Cartee GD. Oki K, et al. J Gerontol A Biol Sci Med Sci. 2020 Jan 20;75(2):207-217. doi: 10.1093/gerona/gly222. J Gerontol A Biol Sci Med Sci. 2020. PMID: 30272137 Free PMC article. - The Regulation of Lipokines by Environmental Factors.
Hernández-Saavedra D, Stanford KI. Hernández-Saavedra D, et al. Nutrients. 2019 Oct 11;11(10):2422. doi: 10.3390/nu11102422. Nutrients. 2019. PMID: 31614481 Free PMC article. Review. - High intensity interval training alters gene expression linked to mitochondrial biogenesis and dynamics in high fat diet fed rats.
Jahangiri M, Shahrbanian S, Gharakhanlou R. Jahangiri M, et al. Sci Rep. 2025 Feb 14;15(1):5442. doi: 10.1038/s41598-025-86767-5. Sci Rep. 2025. PMID: 39952980 Free PMC article. - Predicting Sprint Potential: A Machine Learning Model Based on Blood Metabolite Profiles in Young Male Athletes.
Chen J, Qian Y, Xu Y. Chen J, et al. Eur J Sport Sci. 2025 Mar;25(3):e12272. doi: 10.1002/ejsc.12272. Eur J Sport Sci. 2025. PMID: 39992201 Free PMC article. - Effects of aging on serum levels of lipid molecular species as determined by lipidomics analysis in Japanese men and women.
Kawanishi N, Kato Y, Yokozeki K, Sawada S, Sakurai R, Fujiwara Y, Shinkai S, Goda N, Suzuki K. Kawanishi N, et al. Lipids Health Dis. 2018 Jun 6;17(1):135. doi: 10.1186/s12944-018-0785-6. Lipids Health Dis. 2018. PMID: 29875018 Free PMC article.
References
- Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60: 2588–2597, 2011. doi:10.2337/db10-1221. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous