Serotonin Differentially Regulates L5 Pyramidal Cell Classes of the Medial Prefrontal Cortex in Rats and Mice - PubMed (original) (raw)
Serotonin Differentially Regulates L5 Pyramidal Cell Classes of the Medial Prefrontal Cortex in Rats and Mice
Mary C Elliott et al. eNeuro. 2018.
Abstract
The prefrontal cortex receives a dense serotonergic innervation that plays an important role in its regulation. However, how serotonin regulates different pyramidal and interneuron cell classes in this area is incompletely understood. Previous work in rats has shown that serotonin differentially regulates two classes of pyramidal cells in layer 5. It excites one class by activating 5-HT2A receptors, whereas it more subtly modulates the integrative properties of the other by co-activating 5-HT1A and 5-HT2A receptors. Here we have used electrophysiological recordings, combined with retrograde labeling and morphological reconstruction, to show that the first cell class corresponds to long range corticofugal neurons and the second corresponds to intratelencephalic neurons. These results suggest that, in rats, serotonin facilitates subcortical output while more subtly modulating cortico-cortical and cortico-striatal output. Interestingly, these results obtained in rats differ from those previously reported for mouse prefrontal cortex. Therefore we reinvestigated the effects of serotonin in mice and confirmed that serotonin predominantly activates inhibitory 5-HT1A receptors on long-range corticofugal cells. Thus serotonin exerts opposite effects on these cells in rats and mice. Finally, we determined whether cortical serotonin responsiveness in mice is regulated during development. Serotonin elicited predominantly depolarizing inward current responses during the early postnatal period, whereas inhibitory 5-HT1A receptor-mediated responses did not become evident until the end of the second postnatal week. These results reveal commonalities as well as unexpected differences in the serotonergic regulation of long-range corticofugal and intratelencephalic neurons of layer 5 in rat and mouse.
Keywords: Corticofugal; development; intratelencephalic; prefrontal cortex; pyramidal cell; serotonin.
Figures
Figure 1.
Electrophysiological properties define two pyramidal cell types in L5 of the rat mPFC. One of these corresponds to regular spiking neurons exhibiting limited firing frequency adaptation in response to constant depolarizing current injection (A1). These cells exhibit a prominent ImAHP that is partially inhibited by apamin (100 nM, A2) and a relatively fast activating Ih (A3) under voltage clamp. We have termed these cells type I, weakly adapting pyramidal cells. The second cell type exhibits strong spike frequency adaptation in response to constant depolarizing current injection (B1), a prominent apamin-insensitive IsAHP (B2), and slow-activating Ih (B3). We have termed this second cell type II, strongly adapting pyramidal cells. A2, A3, B2, B3, Vh = –60 mV. Consistent with this typology, a histogram of spike frequency accommodation characteristics (as defined by the accommodation index) suggests a bimodal distribution (C).
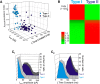
Figure 2.
Sorting of L5 pyramidal cells. A, Plotting the amplitude of IsAHP, the time constant of Ih and the AI cluster the cells in our sample into what appear to be two largely nonoverlapping subsets. B, Autoclass, an unsupervised Bayesian classifier, also sorted the cells in our sample into two classes with the vast majority of cells sorting unambiguously to one of the two classes. The heat map depicts the probability that each cell tested in the current sample belongs to the type I or the type II classes. Probability is color-coded in a green-red scale as indicated in the side bar. After sorting, type I and type II cells, considered as groups, can be seen to differ in terms of the amplitude distribution for IsAHP (C1) and the distribution of time constant for Ih (C2). C1-C2: n = 100 cells.
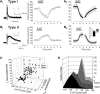
Figure 3.
Effect of serotonin on type I and type II pyramidal cells of L5. A1, Administration of serotonin (10 μ
m
) to an electrophysiologically identified type I pyramidal cell of L5 elicits an inward current that recovers after removal of serotonin from the bath. _A_2, Summary plot illustrating the mean current (± SEM) elicited by serotonin on 38 type I cells of L5. Vh = –60 mV. B1, Administration of serotonin (10 μ
m
) to an electrophysiologically identified type II cell of L5 elicits an outward current that recovers on removal of serotonin from the bath. B2, Summary plot illustrating the mean current (± SEM) elicited by serotonin on 59 type II cells tested. Vh = –60 mV. Notice that the initial outward current is followed a net inward current as previously reported for many of these cells (Araneda and Andrade, 1991). B2 inset, Bar graph illustrating the peak initial current induced by serotonin. *, p < 0.001. C, 3D plot graphing the time constant of Ih, the amplitude of IsAHP, and the amplitude of the initial current induced by serotonin in the cell sampled in the current work. D, Amplitudes distribution for the initial serotonin-induced current in type I and type II cells.
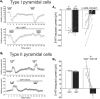
Figure 4.
Pharmacology of serotonin responses in type I and type II L5 pyramidal cells of rat mPFC. A1, Top, two consecutive applications of serotonin (10 μ
m
) to a type I pyramidal cell results in comparable inward currents indicating minimal desensitization of the response. Bottom, administration of the selective 5-HT2A receptor antagonist MDL100 907 (300 n
m
) between serotonin application inhibits the effect of a second serotonin application. A2, Summary plot illustrating the effect of MDL100907 on the inward current elicit by serotonin on type I pyramidal cells. B1, Top, two consecutive applications of serotonin (10 μ
m
) to a type II pyramidal cell elicit comparable outward currents. Bottom, administration of the selective 5-HT1A antagonist WAY100135 (3 μ
m
) between serotonin application blocks the effect of a second application of serotonin. B2, Summary plot illustrating the effect of WAY100135 on the outward current elicited by serotonin in type II pyramidal cells.
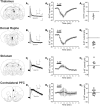
Figure 5.
Effect of serotonin on retrogradely labeled L5 pyramidal neurons. Thalamus. A1, IsAHP and Ih recorded from a L5 neuron retrogradely labeled from the thalamus. Ih scale: 200 pA, 1 s. IsAHP scale: 50 pA, 250 ms. The scale applies to panels A1–D1. A2, Summary plot illustrating the mean current (± SEM) elicited by serotonin (10 μ
m
) in neurons retrogradely labeled from the thalamus (n = 5 cells). A3, Graph illustrating the peak serotonin current recorded in these cells. Dorsal raphe. B1, IsAHP and Ih recorded from a L5 neuron retrogradely labeled from the dorsal raphe. B2, Summary plot illustrating the mean current (± SEM) elicited by serotonin (10 μ
m
) in neurons retrogradely labeled from the dorsal raphe (n = 9 cells). B3, Graph illustrating the peak serotonin current recorded in these cells. Striatum. C1, IsAHP and Ih recorded from a L5 neuron retrogradely labeled from the striatum. C2, Summary plot illustrating the mean current (± SEM) elicited by serotonin (10 μ
m
) in neurons retrogradely labeled from the striatum (n = 8 cells). C3, Graph illustrating the peak serotonin current recorded in these cells. Contralateral PFC. D1, IsAHP and Ih recorded from a L5 neuron retrogradely labeled from the contralateral PFC. D2, Summary plot illustrating the mean current (± SEM) elicited by serotonin (10 μ
m
) in neurons retrogradely labeled from the contralateral PFC (n = 11 cells). D3, Graph illustrating the peak serotonin current recorded in these cells. The cells illustrated in panels A–D were assigned a probability of at least 95% for belonging to the type I class (A and B) or the type II class (C and D) by the Bayesian classifier.
Figure 6.
Neuronal morphology of type I and type II neurons. A, Reconstruction of an electrophysiological and pharmacologically identified type I neuron. Neuron reconstruction scale: 100 μm, Ih inset scale, 500 ms, 200 pA. B, Reconstruction of an electrophysiological and pharmacologically identified type II neuron. Neuron reconstruction scale: 100 μm, Ih inset scale, 500 ms, 200 pA. C, D, Scholl plots for the apical (filled circle) and basal (hollow circles) dendrites. Error bars depict the SEM. Branch order cumulative histogram for type I and type II pyramidal cells of L5. Panels C–E depict results derived from 5 type I and 7 type II neurons.
Figure 7.
Type I and type II cells in L5 of the mouse mPFC. A1, A2, Electrophysiological characterization and response to serotonin administration (10 μ
m
) in two L5 pyramidal cells, one classified as a type I and the other as type II by the Bayesian sorter. B, Heat map depicting the probability that each of the 72 cells tested belongs to the type I and type II classes as determined by Autoclass. The probability for each cell is color-coded as depicted in the scale bar. C, Summary graph plotting the amplitude of the peak serotonin-induced currents in the cells sampled. D, Effect of serotonin on IsAHP and IsADP in type I and type II cells. Traces were baseline subtracted for clarity. The type I cell responded to serotonin with a 69-pA outward current and the type II cell with a –24-pA inward current. Overall, in this group of cells, serotonin (10–30 μ
m
) elicited a –14 ± 6.5-pA inward shift in the aftercurrent in type I cells and –27 ± 3.5 pA in type II cells (p < 0.01).
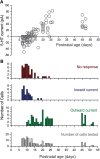
Figure 8.
Effects of serotonin on mouse L5 pyramidal neurons during the early postnatal period. A, Graph plotting the peak initial current elicited by serotonin on pyramidal cells of L5 as a function of postnatal age. B, Histograms depicting the number of cells exhibiting no response, an inward current, and outward current as a function of postnatal age, as well as the number of cells tested at each age.
Similar articles
- Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors.
Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Béïque JC, et al. J Neurosci. 2004 May 19;24(20):4807-17. doi: 10.1523/JNEUROSCI.5113-03.2004. J Neurosci. 2004. PMID: 15152041 Free PMC article. - Serotonergic Suppression of Mouse Prefrontal Circuits Implicated in Task Attention.
Tian MK, Schmidt EF, Lambe EK. Tian MK, et al. eNeuro. 2016 Nov 8;3(5):ENEURO.0269-16.2016. doi: 10.1523/ENEURO.0269-16.2016. eCollection 2016 Sep-Oct. eNeuro. 2016. PMID: 27844060 Free PMC article. - Mechanisms Underlying Serotonergic Excitation of Callosal Projection Neurons in the Mouse Medial Prefrontal Cortex.
Stephens EK, Baker AL, Gulledge AT. Stephens EK, et al. Front Neural Circuits. 2018 Jan 18;12:2. doi: 10.3389/fncir.2018.00002. eCollection 2018. Front Neural Circuits. 2018. PMID: 29422840 Free PMC article. - Serotonergic regulation of neuronal excitability in the prefrontal cortex.
Andrade R. Andrade R. Neuropharmacology. 2011 Sep;61(3):382-6. doi: 10.1016/j.neuropharm.2011.01.015. Epub 2011 Jan 18. Neuropharmacology. 2011. PMID: 21251917 Free PMC article. Review. - [Serotonergic control of prefrontal cortex].
Puig MV, Celada P, Artigas F. Puig MV, et al. Rev Neurol. 2004 Sep 16-30;39(6):539-47. Rev Neurol. 2004. PMID: 15467993 Review. Spanish.
Cited by
- 5-HT2A receptor dysregulation in a schizophrenia relevant mouse model of NMDA receptor hypofunction.
Nakao K, Singh M, Sapkota K, Fitzgerald A, Hablitz JJ, Nakazawa K. Nakao K, et al. Transl Psychiatry. 2022 Apr 22;12(1):168. doi: 10.1038/s41398-022-01930-0. Transl Psychiatry. 2022. PMID: 35459266 Free PMC article. - The Anatomy of Inference: Generative Models and Brain Structure.
Parr T, Friston KJ. Parr T, et al. Front Comput Neurosci. 2018 Nov 13;12:90. doi: 10.3389/fncom.2018.00090. eCollection 2018. Front Comput Neurosci. 2018. PMID: 30483088 Free PMC article. Review. - A Dendrite-Focused Framework for Understanding the Actions of Ketamine and Psychedelics.
Savalia NK, Shao LX, Kwan AC. Savalia NK, et al. Trends Neurosci. 2021 Apr;44(4):260-275. doi: 10.1016/j.tins.2020.11.008. Epub 2020 Dec 21. Trends Neurosci. 2021. PMID: 33358035 Free PMC article. Review. - Pyramidal cell types and 5-HT2A receptors are essential for psilocybin's lasting drug action.
Shao LX, Liao C, Davoudian PA, Savalia NK, Jiang Q, Wojtasiewicz C, Tan D, Nothnagel JD, Liu RJ, Woodburn SC, Bilash OM, Kim H, Che A, Kwan AC. Shao LX, et al. bioRxiv [Preprint]. 2024 Nov 3:2024.11.02.621692. doi: 10.1101/2024.11.02.621692. bioRxiv. 2024. PMID: 39554087 Free PMC article. Updated. Preprint. - Neocortical layer 5 subclasses: From cellular properties to roles in behavior.
Moberg S, Takahashi N. Moberg S, et al. Front Synaptic Neurosci. 2022 Oct 28;14:1006773. doi: 10.3389/fnsyn.2022.1006773. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36387773 Free PMC article. Review.
References
- Andrade R, Beck SG (2010) Cellular effects of serotonin in the CNS In: Handbook of the Behavioral Neurobiology of Serotonin, First Edition (Muller CP, Jacobs BL, eds), pp. 219–231. London: Academic Press/Elsevier.
- Araneda R, Andrade R (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40:399–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources