DNA repair deficiency sensitizes lung cancer cells to NAD+ biosynthesis blockade - PubMed (original) (raw)
. 2018 Apr 2;128(4):1671-1687.
doi: 10.1172/JCI90277. Epub 2018 Mar 19.
Mehdi Touat 1 2 3, Nicolas Dorvault 1 4, Roman M Chabanon 1 4, Marlène Garrido 1 4, Daphné Morel 1 4, Dragomir B Krastev 5, Ludovic Bigot 1, Julien Adam 1 6, Jessica R Frankum 5, Sylvère Durand 7, Clement Pontoizeau 8 9 [ 10](#full-view-affiliation-10 "Inserm U1163, Institut Imagine, Equipe "Génétique des Maladies Mitochondriales" and Paris Descartes University, Paris, France."), Sylvie Souquère 11, Mei-Shiue Kuo 1, Sylvie Sauvaigo 12, Faraz Mardakheh 13, Alain Sarasin 14, Ken A Olaussen 1 15, Luc Friboulet 1, Frédéric Bouillaud 16, Gérard Pierron 11, Alan Ashworth 17, Anne Lombès 16, Christopher J Lord 5, Jean-Charles Soria 1 2 15, Sophie Postel-Vinay 1 2 4 5
Affiliations
- PMID: 29447131
- PMCID: PMC5873862
- DOI: 10.1172/JCI90277
DNA repair deficiency sensitizes lung cancer cells to NAD+ biosynthesis blockade
Mehdi Touat et al. J Clin Invest. 2018.
Abstract
Synthetic lethality is an efficient mechanism-based approach to selectively target DNA repair defects. Excision repair cross-complementation group 1 (ERCC1) deficiency is frequently found in non-small-cell lung cancer (NSCLC), making this DNA repair protein an attractive target for exploiting synthetic lethal approaches in the disease. Using unbiased proteomic and metabolic high-throughput profiling on a unique in-house-generated isogenic model of ERCC1 deficiency, we found marked metabolic rewiring of ERCC1-deficient populations, including decreased levels of the metabolite NAD+ and reduced expression of the rate-limiting NAD+ biosynthetic enzyme nicotinamide phosphoribosyltransferase (NAMPT). We also found reduced NAMPT expression in NSCLC samples with low levels of ERCC1. These metabolic alterations were a primary effect of ERCC1 deficiency, and caused selective exquisite sensitivity to small-molecule NAMPT inhibitors, both in vitro - ERCC1-deficient cells being approximately 1,000 times more sensitive than ERCC1-WT cells - and in vivo. Using transmission electronic microscopy and functional metabolic studies, we found that ERCC1-deficient cells harbor mitochondrial defects. We propose a model where NAD+ acts as a regulator of ERCC1-deficient NSCLC cell fitness. These findings open therapeutic opportunities that exploit a yet-undescribed nuclear-mitochondrial synthetic lethal relationship in NSCLC models, and highlight the potential for targeting DNA repair/metabolic crosstalks for cancer therapy.
Keywords: DNA repair; Lung cancer; Mitochondria; Oncology.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
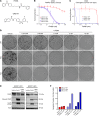
Figure 1. SILAC-based proteomic analysis identifies NAMPT decrease as a potentially targetable node in ERCC1-deficient clones.
(A) Experimental workflow for quantitative proteomic analysis using SILAC. Two independent experiments in the ERCC1-WT and ERCC1-KO cell lines from the A549 model with inverted labeling of the cell lines (forward and reverse experiments) were set up and run in parallel in order to limit the number of false-positive hits by having an internal control (n = 2 independent samples for each model). Protein lysates were mixed in a 1:1 proportion between the heavy- and light-labeled cells. After protein digestion and off-gel fractionation, fractions were analyzed by LC-MS/MS, and data analysis was performed by MaxQuant. (B) Scatter plot of the ratio of heavy over light amino acids normalized to the reverse (R) or forward (F) experiment, following a log2 normalization. Hits reaching significance (Benjamini-Hochberg adjusted P value < 0.05) in both experiments are depicted in red. FP, false-positive. (C) Canonical NAD+ biosynthetic pathways. In humans, most NAD+ is synthesized from nicotinamide (NAm) through the salvage (recycling) pathway. NAMPT (NAm phosphoribosyltransferase) catalyzes the rate-limiting step in this pathway. Sirtuins (SIRTs) and poly(ADP-ribose) polymerase (PARPs) enzymes catalyze reactions that consume NAD+. The reaction scheme is based on the Biocyc pathway map for homo sapiens NAD+ biosynthesis (
), and adapted from Kim et al (15). NAPRT, nicotinic acid phosphoribosyltransferase; QPRT, quinolinic acid phosphoribosyltransferase; NMNATs, NMN adenylyltransferases; NADS, NAD+ synthetase.
Figure 2. ERCC1 expression correlates with NAMPT expression in human adenocarcinoma cell lines and patient samples.
(A and B) Representative Western blot of NAMPT and ERCC1 protein expression in the ERCC1-deficient isogenic models derived from the A549 (A) and H1975 (B) NSCLC cell lines. Data were replicated in 3 independent experiments. (C) NAMPT mRNA levels in ERCC1-WT, ERCC1-Hez, and ERCC1-KO cell lines from the A549 model. Data are box plots of NAMPT mRNA levels (± minimal and maximal values) with n = 4 independent experiments for each model. Statistical analyses are indicated (Kruskal-Wallis 1-way ANOVA adjusted using Dunn’s multiple comparison test). (D) ERCC1 and NAMPT immunohistochemical stainings in human adenocarcinoma samples. Data are box plots (± minimal and maximal values) showing NAMPT protein expression according to ERCC1 expression in n = 55 tumor samples. Statistical analyses are indicated (Mann-Whitney U test). (E) Representative images of ERCC1 and NAMPT immunohistochemical stainings in human tumor samples. Scale bar: 50 μm.
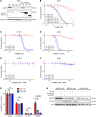
Figure 3. NAMPT decrease in ERCC1-deficient clones leads to exquisite sensitivity to NAMPT inhibitors.
(A) Chemical structures of NAMPT inhibitors FK866 and GNE-617. (B and C) Survival curves of the A549 ERCC1 isogenic model on FK866 exposure in short-term assay (B) and long-term colony formation assay (C). Data are mean surviving fractions ± SD from 1 of 3 independent experiments. (D) Pictures of the colony formation assay in the A549 ERCC1 isogenic model after 14 days of treatment with vehicle or FK866. (E) Representative Western blot of PARP and LC3 protein expression in A549 ERCC1-proficient and ERCC1-deficient cells treated with vehicle or FK866. Thapsigargin (THAPS) was used as positive control for apoptosis and induction of autophagy. Data are from 1 experiment. (F) Fraction of annexin V–positive (both 7-AAD–negative or 7-AAD–positive) cells determined by flow cytometry analysis after 5 days of exposure with FK866 in A549 ERCC1-proficient and ERCC1-deficient cells. Data are from 1 experiment.
Figure 4. Exquisite sensitivity to NAMPT inhibition is a primary effect of ERCC1 deficiency.
(A) Representative Western blot of NAMPT and ERCC1 protein expression and (B) survival experiment on FK866 exposure according to the reintroduction of the 4 alternative ERCC1 isoforms. (C) Survival experiment on FK866 exposure of the H1975 ERCC1-isogenic model. (D) Survival experiment on GNE-617 exposure of the A549 ERCC1-isogenic model. (E and F) Rescue of FK866 toxicity by the administration of nicotinamide mononucleotide (NMN) 100 μM in the H1975 (E) and A549 (F) ERCC1-isogenic models. Data for B–F are mean surviving fraction ± SD from 1 experiment. (G) Cell viability after acute NAMPT silencing by siRNA in A549 ERCC1-proficient and ERCC1-deficient cells. Toxicity of PLK1 silencing by siRNA was used as a positive control for cell death induction. Data are mean viability ± SD from 1 representative experiment. Statistical analyses are indicated (Mann-Whitney U test corrected for multiple comparisons). NS indicates no statistically significant difference. (H) Representative Western blot of NAMPT and ERCC1 protein expression after NAMPT silencing by siRNA in A549 ERCC1-proficient and ERCC1-deficient cells. For A–H, data were replicated in at least 3 independent experiments.
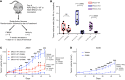
Figure 5. NAMPT inhibition is synthetic lethal with ERCC1 deficiency in in vivo models of NSCLC.
(A) Experimental workflow for the in vivo experiments. Treatment was initiated when tumors reached a volume of 100 mm3. Tumor-bearing mice (n = 8 per group) were randomized to receive 30 mg/kg/day FK866 or vehicle for 6 weeks following a 4 days on, 3 days off schedule. (B) Changes in tumor volume after 44 days of treatment with FK866 in ERCC1-proficient and ERCC1-deficient tumors in nude mice. Data are mean tumor volume ± SD (n = 8 mice per group). Statistical analyses are indicated (2-way ANOVA adjusted for multiple comparisons using Bonferroni’s post hoc test). (C and D) Tumor growth on FK866 treatment in the A549 ERCC1-WT and ERCC1-KO1 models (C), and in the ERCC1-KO2 model (D). Data represent mean tumor volume ± SEM (n = 8 mice per group). Statistical analyses are indicated (2-way ANOVA adjusted for multiple comparisons using Bonferroni’s post hoc test). *P < 0.05, **P < 0.01, ***P < 0.0001.
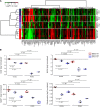
Figure 6. ERCC1-deficient cells present a characteristic metabolic profile with alterations in the NAD+ biosynthesis pathway.
(A) Heatmap depicting relative abundance of 159 metabolites (annotation level 1, see Methods) determined by metabolomic profiling in the ERCC1-WT (n = 5 independent samples), ERCC1-Hez (n = 3), and ERCC1-KO (n = 3) cell lines from the A549 model. Samples (rows) and metabolites (columns) were reordered by hierarchical clustering using the Ward algorithm on their respective Euclidean distance matrices. (B) Box plots showing the relative abundance of central metabolites of the NAD+ pathway (NAD+, NADH, NADP, and nicotinamide) across the ERCC1-WT (n = 5 independent samples), ERCC1-Hez (n = 3), and ERCC1-KO (n = 3) cell lines with and without treatment by FK866. Data were centered on the ERCC1-WT vehicle group for comparative purposes. All statistical analyses and data representation were performed on preprocessed, log2-transformed and imputed data and reported as such without back transformation. Moderated statistics were used for differential analysis. Levels of significance were denoted as adjusted Benjamini-Hochberg P values to control the FDR.
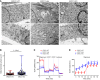
Figure 7. ERCC1-deficient cells present an abnormal mitochondrial structure associated with decreased respiratory capacity and increased glycolysis.
(A) Representative transmission electron microscopy (TEM) pictures of the A549 ERCC1-WT, ERCC1-Hez, and ERCC1-KO cell lines. m, mitochondria. Scale bars: 1 μm. (B) Measurements of mitochondrial area using TEM in A549 ERCC1-deficient and ERCC1-proficient cells. Data are box plots (± minimal and maximal values) from n = 87–122 mitochondria per group. Statistical analyses are indicated (Mann-Whitney U test). (C and D) OCR and ECAR of intact A549 ERCC1-WT, ERCC1-Hez, and ERCC1-KO cells measured in real time with the Seahorse XF96 analyzer. Indices of glycolysis and mitochondrial function were measured after sequential injections of oligomycin (1 μg/ml final), CCCP (first 0.25 μM then 0.50 μM), and antimycin A (1 μg/ml). Data are mean ± SD from 1 experiment. OCR, oxygen consumption rate; ECAR, extracellular acidification rate; CCCP, carbonyl cyanide 3-chlorophenylhydrazone. For A–D, data were replicated in 3 independent experiments.
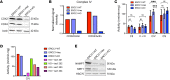
Figure 8. ERCC1-KO cells have a decreased mitochondrial complex IV activity associated with decreased SIRT1 protein expression.
Representative Western blot (A) and quantification (B) of COX2 and COX4 protein expression in A549 ERCC1-proficient and ERCC1-deficient cell lines. Data were replicated in 3 independent experiments. (C) Enzymatic activities of mitochondrial complexes measured using respiratory chain spectrophotometric assay in A549 ERCC1-proficient and ERCC1-deficient cell lines (n = 3 independent samples per group). Data are mean enzymatic activity ± SD. Statistical analyses are indicated (2-way ANOVA adjusted using Tukey’s multiple comparison test). NS indicates no statistically significant difference. ***P < 0.0001. (D) Enzymatic activity of mitochondrial complex IV in A549 ERCC1-proficient and ERCC1-deficient models in which each ERCC1 isoform has been stably reintroduced. Data are from 1 experiment. (E) Western blot of NAMPT and SIRT1 protein expression in A549 ERCC1-WT, ERCC1-Hez, and ERCC1-KO cells. Data are from 1 experiment.
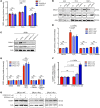
Figure 9. Loss of ERCC1 results in increased ADP-ribosylation and a gradual decrease in NAMPT expression.
(A) ADP-ribosylation (ADPr) levels were measured by immunofluorescence in A549 cells not treated (NT) or treated with control (scramble siRNA) or ERCC1 siRNA (siERCC1). H2O2 exposure was used for inducing DNA damage. Olaparib was used as control for abrogation of PARP1-related ADP-ribosylation activity. Data are mean ADP-ribosylation activity ± SD from 1 of 3 independent experiments. (B) Representative Western blot of ERCC1 and NAMPT protein expression after acute ERCC1 silencing using siRNA in A549 cells. NT, nontransfected. Data were replicated in 2 independent experiments. (C) Western blot of ERCC1 and NAMPT protein expression after sequential transfections with shERCC1-coding viral particles in A549 cells. V1, V2, V3 indicate infections 1, 2, and 3, respectively, which were performed 10–15 days apart each. Data are from 1 experiment. (D and E) ADP-ribosylation levels were measured by immunofluorescence in the A549 isogenic model in the absence (D) or presence (E) of NMN supplementation. Data are mean ADP-ribosylation activity ± SD from 1 of 2 independent experiments. (F) Variations in NAMPT mRNA levels in A549 ERCC1-WT, ERCC1-Hez, and ERCC1-KO cell lines following 3-day exposure to nontoxic concentrations of the SIRT1 activator STR2104. Data represent mean 2–ΔΔCt ± SD from 1 experiment. Statistical analyses are indicated (2-way ANOVA adjusted using Sidak’s multiple comparison test). (G) Representative Western blot of NAMPT protein expression after 4-day exposure to nontoxic concentrations of STR2104. Data were replicated in 2 independent experiments. For A, D, E, statistical analyses are indicated (2-way ANOVA adjusted for multiple comparisons using Tukey’s t test).
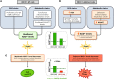
Figure 10. Proposed model for the synthetic lethality between NAMPT inhibition and ERCC1 deficiency in NSCLC cells.
(A) ERCC1-proficient cells have a proficient DNA damage repair status and no metabolic alteration in the NAD+ biosynthesis pathway. (B) Loss of ERCC1 induces decreased NER activity and dependency upon PARP1 activation for the repair of DNA lesions, thereby causing NAD+ consumption. Chronic ERCC1 deficiency/PARP1 activation is associated with metabolic defects including decreased NAD+ and NAMPT levels, as well as alterations in the mitochondrial respiratory chain. In this scenario, NAD+ levels are low, but remain above a threshold that allows ERCC1-deficient cells to survive and proliferate. (C) Treatment with NAMPT inhibitors blocks the NAD+ recycling pathway and causes an acute drop in NAD+ levels. In ERCC1-proficient cells, NAD+ levels remain above a critical threshold compatible with cell survival. In ERCC1-deficient cells, this acute drop in NAD+ levels outstrips the cellular NAD+ replenishment capacities and decompensates a fragile equilibrium; NAD+ levels drop below a threshold compatible with cell survival, which triggers cell death. In this model, NAMPT and ERCC1 are synthetic lethal, as the combination of ERCC1 deficiency with NAMPT inhibition causes cell death, whereas each perturbation in isolation (i.e., ERCC1 deficiency or NAMPT inhibition) does not. Arrows represent a relationship and not a demonstrated causality link. DDR, DNA damage response; ERCC1-def, ERCC1-deficient; NER, nucleotide excision repair.
Similar articles
- A high-throughput screen identifies PARP1/2 inhibitors as a potential therapy for ERCC1-deficient non-small cell lung cancer.
Postel-Vinay S, Bajrami I, Friboulet L, Elliott R, Fontebasso Y, Dorvault N, Olaussen KA, André F, Soria JC, Lord CJ, Ashworth A. Postel-Vinay S, et al. Oncogene. 2013 Nov 21;32(47):5377-87. doi: 10.1038/onc.2013.311. Epub 2013 Aug 12. Oncogene. 2013. PMID: 23934192 - Inhibition of p38 MAPK-dependent excision repair cross-complementing 1 expression decreases the DNA repair capacity to sensitize lung cancer cells to etoposide.
Tsai MS, Weng SH, Chen HJ, Chiu YF, Huang YC, Tseng SC, Kuo YH, Lin YW. Tsai MS, et al. Mol Cancer Ther. 2012 Mar;11(3):561-71. doi: 10.1158/1535-7163.MCT-11-0684. Epub 2011 Nov 3. Mol Cancer Ther. 2012. PMID: 22053010 - EGFR exon 19-deletion aberrantly regulate ERCC1 expression that may partly impaired DNA damage repair ability in non-small cell lung cancer.
Zhang L, Pradhan B, Guo L, Meng F, Zhong D. Zhang L, et al. Thorac Cancer. 2020 Feb;11(2):277-285. doi: 10.1111/1759-7714.13253. Epub 2019 Dec 25. Thorac Cancer. 2020. PMID: 31875360 Free PMC article. - Pharmacogenetics of platinum-based chemotherapy in non-small cell lung cancer: predictive validity of polymorphisms of ERCC1.
Hamilton G, Rath B. Hamilton G, et al. Expert Opin Drug Metab Toxicol. 2018 Jan;14(1):17-24. doi: 10.1080/17425255.2018.1416095. Epub 2017 Dec 18. Expert Opin Drug Metab Toxicol. 2018. PMID: 29226731 Review.
Cited by
- NAMPT-Driven M2 Polarization of Tumor-Associated Macrophages Leads to an Immunosuppressive Microenvironment in Colorectal Cancer.
Hong SM, Lee AY, Kim BJ, Lee JE, Seon SY, Ha YJ, Ng JT, Yoon G, Lim SB, Morgan MJ, Cha JH, Lee D, Kim YS. Hong SM, et al. Adv Sci (Weinh). 2024 Apr;11(14):e2303177. doi: 10.1002/advs.202303177. Epub 2024 Feb 2. Adv Sci (Weinh). 2024. PMID: 38308188 Free PMC article. - ATM Inhibitor Suppresses Gemcitabine-Resistant BTC Growth in a Polymerase θ Deficiency-Dependent Manner.
Pan YR, Wu CE, Yeh CN. Pan YR, et al. Biomolecules. 2020 Nov 9;10(11):1529. doi: 10.3390/biom10111529. Biomolecules. 2020. PMID: 33182492 Free PMC article. - XRCC1 counteracts poly(ADP ribose)polymerase (PARP) poisons, olaparib and talazoparib, and a clinical alkylating agent, temozolomide, by promoting the removal of trapped PARP1 from broken DNA.
Hirota K, Ooka M, Shimizu N, Yamada K, Tsuda M, Ibrahim MA, Yamada S, Sasanuma H, Masutani M, Takeda S. Hirota K, et al. Genes Cells. 2022 May;27(5):331-344. doi: 10.1111/gtc.12929. Epub 2022 Mar 1. Genes Cells. 2022. PMID: 35194903 Free PMC article. - Advances in NAD-Lowering Agents for Cancer Treatment.
Ghanem MS, Monacelli F, Nencioni A. Ghanem MS, et al. Nutrients. 2021 May 14;13(5):1665. doi: 10.3390/nu13051665. Nutrients. 2021. PMID: 34068917 Free PMC article. Review. - Beyond Energy Metabolism: Exploiting the Additional Roles of NAMPT for Cancer Therapy.
Heske CM. Heske CM. Front Oncol. 2020 Jan 17;9:1514. doi: 10.3389/fonc.2019.01514. eCollection 2019. Front Oncol. 2020. PMID: 32010616 Free PMC article. Review.
References
- Global Burden of Disease Cancer Collaboration, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous