Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins - PubMed (original) (raw)
Review
Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins
Inês Vieira da Silva et al. Cell Mol Life Sci. 2018 Jun.
Abstract
The metabolic syndrome (MetS) includes a group of medical conditions such as insulin resistance (IR), dyslipidemia and hypertension, all associated with an increased risk for cardiovascular disease. Increased visceral and ectopic fat deposition are also key features in the development of IR and MetS, with pathophysiological sequels on adipose tissue, liver and muscle. The recent recognition of aquaporins (AQPs) involvement in adipose tissue homeostasis has opened new perspectives for research in this field. The members of the aquaglyceroporin subfamily are specific glycerol channels implicated in energy metabolism by facilitating glycerol outflow from adipose tissue and its systemic distribution and uptake by liver and muscle, unveiling these membrane channels as key players in lipid balance and energy homeostasis. Being involved in a variety of pathophysiological mechanisms including IR and obesity, AQPs are considered promising drug targets that may prompt novel therapeutic approaches for metabolic disorders such as MetS. This review addresses the interplay between adipose tissue, liver and muscle, which is the basis of the metabolic syndrome, and highlights the involvement of aquaglyceroporins in obesity and related pathologies and how their regulation in different organs contributes to the features of the metabolic syndrome.
Keywords: Aquaglyceroporins; Aquaporins; Glycerol; Metabolic syndrome; Obesity.
Figures
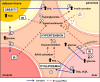
Fig. 1
MetS results from the interplay between adipose tissue, skeletal muscle, liver and pancreas. Obesity development and adipose tissue dysfunction lead to high levels of circulating FFA (1). Decreased insulin sensitivity in liver and muscle results in impaired glucose uptake (2) and hyperglycemia (3). Over-stimulation of insulin release by endocrine pancreas (4) ends in hyperinsulinemia (5) and consequently IR. In the liver, glucose production, lipid storage in the form of triacylglycerol and VLDL secretion are increased (6) contributing to dyslipidemia (7). Lack of insulin action combined with high levels of FFA activates the RAAS, leading to vasoconstriction and causing hypertension (8). FFA free fatty acids, IR insulin resistance, RAAS renin angiotensin aldosterone system, TAG triacylglycerols, VLDL very-low-density lipoproteins
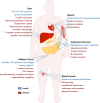
Fig. 2
The most significant events associated with MetS. Summary of the most significant events in healthy (blue) and in disease (red) conditions affecting the main tissues implicated in MetS (adipose tissue, liver, muscle, endocrine pancreas and vessels)
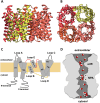
Fig. 3
General structure of aquaporins. a Side and b intracellular views of the homotetrameric representation of the glycerol channel GlpF based on its X-ray structure (PDB code: 1FX8). Figures were generated with UCSF Chimera software. c Representation of AQP membrane topography, showing the monomer comprising six membrane-spanning α-helices (H1–6) connected by five loops (A–E), the conserved asparagine–proline–alanine (NPA) motifs embed in the membrane. In the functional monomer, the hydrophilic loops B and E are bent back into the cavity formed by the helices. The two loops meet in the middle to form the water-selective gate that contains two consensus NPA motifs (Asn–Pro–Ala). The hydrogen bonding properties of the polar side groups of the two Asn residues are thought to constitute the permeation barrier. d Illustration of water and glycerol molecules permeating the aquaporin pore, with the two selectivity filters ar/R and NPA represented
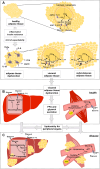
Fig. 4
Involvement of aquaglyceroporins in adipose tissue dysfunction and lipotoxicity in liver and muscle. a In healthy conditions, during fasting when blood nutrient levels are low, TAG hydrolysis in the adipocytes yields FFA and glycerol that is released to the blood stream mainly via AQP7. In the feeding state, when plasma glycerol reaches high concentrations, glycerol is taken up by adipocytes possibly via AQP9, and is converted to TAG that is stored in lipid droplets. When adipose tissue expandability reaches its limits, macrophage infiltration and activation by secretion of proinflammatory molecules (TNFα, IL-1β, IL-6, FFA, leptin) prompt inflammation, loss of insulin sensitivity and adipose tissue dysfunction. In disease, AQP7 downregulation in the subcutaneous adipose tissue results in increase in intracellular glycerol supply for TAG synthesis, which accumulates in lipid droplets with consequent adipocyte hypertrophy. When adipocytes reach their limit of fat accumulation, upregulation of AQP3 and AQP7 in the visceral adipose tissue facilitates the efflux of glycerol into the bloodstream that together with high levels of FFA trigger lipotoxicity in peripheral organs. b In healthy conditions, blood glycerol is taken up by AQP9 in the liver (and possibly by AQP3 and 7) expressed in the basolateral sinusoidal membrane of hepatocytes, and is converted in G3P to be used in gluconeogenesis. In cardiac and skeletal muscle, glycerol is taken up by AQP7 and it is used to generate reductive powder in oxidative phosphorylation for ATP production. c In disease, higher blood glycerol levels stimulate a rise in intracellular G3P in the liver and muscle that can be used for de novo lipogenesis with resultant intracellular TAG accumulation and lipotoxicity. FFA free fatty acids, G3P glycerol-3-phosphate, GK glycerol kinase, TAG triacylglycerols
Similar articles
- Aquaporins in Obesity.
da Silva IV, Soveral G. da Silva IV, et al. Adv Exp Med Biol. 2017;969:227-238. doi: 10.1007/978-94-024-1057-0_15. Adv Exp Med Biol. 2017. PMID: 28258577 Review. - Aquaporins in Obesity.
da Silva IV, Soveral G. da Silva IV, et al. Adv Exp Med Biol. 2023;1398:289-302. doi: 10.1007/978-981-19-7415-1_20. Adv Exp Med Biol. 2023. PMID: 36717502 Review. - Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9.
Maeda N, Funahashi T, Shimomura I. Maeda N, et al. Nat Clin Pract Endocrinol Metab. 2008 Nov;4(11):627-34. doi: 10.1038/ncpendmet0980. Epub 2008 Oct 14. Nat Clin Pract Endocrinol Metab. 2008. PMID: 18852723 Review. - Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control.
Rodríguez A, Catalán V, Gómez-Ambrosi J, Frühbeck G. Rodríguez A, et al. Cell Cycle. 2011 May 15;10(10):1548-56. doi: 10.4161/cc.10.10.15672. Epub 2011 May 15. Cell Cycle. 2011. PMID: 21502813 Free PMC article. - Implications of aquaglyceroporins 7 and 9 in glycerol metabolism and metabolic syndrome.
Maeda N. Maeda N. Mol Aspects Med. 2012 Oct-Dec;33(5-6):665-75. doi: 10.1016/j.mam.2012.02.004. Epub 2012 Mar 8. Mol Aspects Med. 2012. PMID: 22425521 Review.
Cited by
- Combined Systematic Review and Transcriptomic Analyses of Mammalian Aquaporin Classes 1 to 10 as Biomarkers and Prognostic Indicators in Diverse Cancers.
Chow PH, Bowen J, Yool AJ. Chow PH, et al. Cancers (Basel). 2020 Jul 15;12(7):1911. doi: 10.3390/cancers12071911. Cancers (Basel). 2020. PMID: 32679804 Free PMC article. - Aquaglyceroporins Are Differentially Expressed in Beige and White Adipocytes.
da Silva IV, Díaz-Sáez F, Zorzano A, Gumà A, Camps M, Soveral G. da Silva IV, et al. Int J Mol Sci. 2020 Jan 17;21(2):610. doi: 10.3390/ijms21020610. Int J Mol Sci. 2020. PMID: 31963489 Free PMC article. - The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker.
da Silva IV, Garra S, Calamita G, Soveral G. da Silva IV, et al. Biomolecules. 2022 Jun 27;12(7):897. doi: 10.3390/biom12070897. Biomolecules. 2022. PMID: 35883453 Free PMC article. Review. - Recent Update on the Molecular Mechanisms of Gonadal Steroids Action in Adipose Tissue.
Wawrzkiewicz-Jałowiecka A, Lalik A, Soveral G. Wawrzkiewicz-Jałowiecka A, et al. Int J Mol Sci. 2021 May 14;22(10):5226. doi: 10.3390/ijms22105226. Int J Mol Sci. 2021. PMID: 34069293 Free PMC article. Review. - The Effect of Nutritional Ketosis on Aquaporin Expression in Apolipoprotein E-Deficient Mice: Potential Implications for Energy Homeostasis.
da Silva IV, Gullette S, Florindo C, Huang NK, Neuberger T, Ross AC, Soveral G, Castro R. da Silva IV, et al. Biomedicines. 2022 May 18;10(5):1159. doi: 10.3390/biomedicines10051159. Biomedicines. 2022. PMID: 35625895 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical