Frameshift events predict anti-PD-1/L1 response in head and neck cancer - PubMed (original) (raw)
Observational Study
. 2018 Feb 22;3(4):e98811.
doi: 10.1172/jci.insight.98811.
Patrick Lizotte 1 2, Megan Cavanaugh 1 2, Frank C Kuo 3, Priyanka Shivdasani 3, Alexander Frieden 3, Nicole G Chau 1, Jonathan D Schoenfeld 4, Jochen H Lorch 1, Ravindra Uppaluri 5, Laura E MacConaill 3 6, Robert I Haddad 1
Affiliations
- PMID: 29467336
- PMCID: PMC5916245
- DOI: 10.1172/jci.insight.98811
Observational Study
Frameshift events predict anti-PD-1/L1 response in head and neck cancer
Glenn J Hanna et al. JCI Insight. 2018.
Abstract
Programmed cell death protein 1 (PD-1) inhibitors have efficacy in treating squamous cell carcinoma of the head and neck (SCCHN), but objective response rates are low. PD-1 ligand (PD-L1) expression alone is not considered a robust predictor of response and additional biomarkers are needed. This 3-year observational cohort followed 126 SCCHN patients treated with anti-PD-1/L1 therapy. Prior to treatment, 81 (64%) had targeted massively parallel tumor sequencing. Of these, 42 (52%) underwent fluorescence-activated cell sorting and PD-L1 immunohistochemistry for tumor immunoprofiling. Six (5%) complete responses (CRs) and 11 (9%) partial responses (PRs) were observed. Those treated with prior chemotherapy (98, 78%) versus only surgery and/or radiation had longer overall survival (OS) (10 vs. 3 months, P = 0.02). Smokers had a higher total mutational burden (TMB) (P = 0.01). Virus-positive patients had a lower TMB (P < 0.01) and improved OS (P = 0.02). Among virus-negative responders, NOTCH1 and SMARCA4 were more frequently mutated and frameshift events in tumor suppressor genes occurred more frequently (P = 0.03). Higher TMB and CD8+ T cell infiltrates predicted anti-PD-1/L1 benefit (P < 0.01, P < 0.01, respectively) among virus-negative tumors. TIM-3/LAG-3 coexpression with PD-1 was higher on T cells among nonresponders (P = 0.03 and 0.02, respectively). Somatic frameshift events in tumor suppressor genes and higher TMB among virus-negative SCCHN tumors predict anti-PD-1/L1 response.
Keywords: Genetics; Head & neck cancer; Immunotherapy; Molecular genetics; Oncology.
Conflict of interest statement
Conflict of interest: GJH receives institutional research support from BMS and EMD Serono. JDS serves on the scientific advisory board for BMS, Debiopharm, Nanobiotix, and Astra Zeneca. JHL receives institutional research support from Bayer, BMS, Novartis, and Millennium. RIH receives institutional support and consults for BMS, Merck, Astra Zeneca, Pfizer, and Celgene in addition to consulting for Eisai and Genzyme, and is also a member of the National Comprehensive Cancer Network head and neck cancer panel. RU is on the scientific advisory board at Merck.
Figures
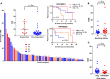
Figure 1. Correlating total mutational burden with response to PD-1/L1 blockade in SCCHN.
(A) Normalized total mutational burden (TMB) among tumor samples (n = 81) arranged from highest to lowest according to anti–PD-1/L1 response. A (+) denotes virus-positive disease. Responders show significantly increased TMB compared with nonresponders. HPV-negative patients with higher TMB demonstrate prolonged overall survival (OS). Greater TMB among (B) former or current (F/C) smokers compared with never (N) smokers, and (C) among patients with virus-mediated [HPV or EBV] disease. SCCHN, squamous cell carcinoma of the head and neck; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; EBV, Epstein-Barr virus; HPV, human papillomavirus. Horizontal bars show median and 95% confidence intervals. Mann-Whitney U test, log-rank testing.
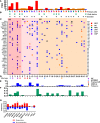
Figure 2. Genomic landscape among anti–PD-1/L1 responders in SCCHN.
(A) Genomic mutational landscape among anti–PD-1/L1 responders (n = 12) and those with SD (n = 20) using a targeted next-generation sequencing platform highlighting total mutational burden (TMB) grouped by high (red, > 10 mutations/Mb), medium (orange, 5–10 mutations/Mb), and low (blue, < 5 mutations/Mb). (B) Primary site of disease (key: top right), viral status (EBV+ or HPV+) and smoking status are shown. (C) The mutational plot shows somatic alterations in order of frequency (highest on top). Somatic mutation key: blue (missense), purple (nonsense), orange (in-frame or frameshift). SA, splice acceptor; SS, splice site; SR, splice region; P, promoter alteration. Only those alterations occurring in 3 or more tumor samples are included in the grid with the exception of genes involved in mismatch repair (MMR). (D) Mutational signatures are displayed (key: lower right). (E) Total indel count (TIC) per tumor sample. (F) Fraction of the genome that is copy-number altered. (G) Proportion of patients with key mutations by response. *P < 0.01 ( χ2 test, 2-sided). SCCHN, squamous cell carcinoma of the head and neck; UV, ultraviolet; CR, complete response; PR, partial response; SD, stable disease; OPC, oropharynx; OC, oral cavity; NPC, nasopharynx; LAR, larynx, hypopharynx; CUT, cutaneous; CUP, carcinoma of unknown primary; APOBEC, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like.
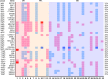
Figure 3. Copy-number alteration events among anti–PD-1/L1 responders in SCCHN.
Copy-number variation (CNV) plot showing losses and gains in order of gene loci among those genes with the greatest frequency of alteration among the cohort (≥8 events). CNV key: light blue (single copy loss), dark blue (homozygous deletion), pink (low copy gain), red (amplification). SCCHN, squamous cell carcinoma of the head and neck; CR, complete response; PR, partial response; SD, stable disease.
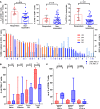
Figure 4. Correlating immunophenotype with response among anti–PD-1/L1 treated patients with SCCHN.
(A) Increased tumor CD8+ T cell infiltration and PD-L1+ infiltrating monocytes among anti–PD-1/L1 responders. Matched PD-1+ CD8+ T cell component (light blue columns). A (+) indicates a virus-mediated tumor. PD-L1 tumor positivity (%) by immunohistochemistry (n = 42). (B) Immune cell phenotyping shows greater effector memory (EM) CD8+ T cells among anti–PD-1/L1 responders (n = 8). (C) Immune checkpoint coexpression appears increased among anti–PD-1/L1 nonresponders (n = 34). Horizontal bars reflect median and 95% confidence intervals. *P < 0.05 determined by Mann-Whitney test, Spearman’s ρ. SCCHN, squamous cell carcinoma of the head and neck; CM, central memory; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Similar articles
- Clinical Implications of Circulating Tumor DNA Tumor Mutational Burden (ctDNA TMB) in Non-Small Cell Lung Cancer.
Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, Cruz MR, Tamragouri K, Rhee K, Mohindra N, Villaflor V, Park W, Lopes G, Giles FJ. Chae YK, et al. Oncologist. 2019 Jun;24(6):820-828. doi: 10.1634/theoncologist.2018-0433. Epub 2019 Mar 13. Oncologist. 2019. PMID: 30867242 Free PMC article. - HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer.
Miyauchi S, Sanders PD, Guram K, Kim SS, Paolini F, Venuti A, Cohen EEW, Gutkind JS, Califano JA, Sharabi AB. Miyauchi S, et al. Cancer Res. 2020 Feb 15;80(4):732-746. doi: 10.1158/0008-5472.CAN-19-1771. Epub 2019 Dec 17. Cancer Res. 2020. PMID: 31848196 Free PMC article. - The effects of checkpoint inhibition on head and neck squamous cell carcinoma: A systematic review.
Ghanizada M, Jakobsen KK, Grønhøj C, von Buchwald C. Ghanizada M, et al. Oral Oncol. 2019 Mar;90:67-73. doi: 10.1016/j.oraloncology.2019.01.018. Epub 2019 Feb 5. Oral Oncol. 2019. PMID: 30846179 - [Current events in immunotherapy for upper aerodigestive tract cancer].
Outh-Gauer S, Le Tourneau C, Broudin C, Scotte F, Roussel H, Hans S, Mandavit M, Tartour E, Badoual C. Outh-Gauer S, et al. Ann Pathol. 2017 Feb;37(1):79-89. doi: 10.1016/j.annpat.2016.12.013. Epub 2017 Jan 19. Ann Pathol. 2017. PMID: 28111039 Review. French. - Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck.
Saada-Bouzid E, Peyrade F, Guigay J. Saada-Bouzid E, et al. Curr Opin Oncol. 2019 May;31(3):146-151. doi: 10.1097/CCO.0000000000000522. Curr Opin Oncol. 2019. PMID: 30893146 Review.
Cited by
- Personalized Treatment Strategies via Integration of Gene Expression Biomarkers in Molecular Profiling of Laryngeal Cancer.
Maniaci A, Giurdanella G, Chiesa Estomba C, Mauramati S, Bertolin A, Lionello M, Mayo-Yanez M, Rizzo PB, Lechien JR, Lentini M. Maniaci A, et al. J Pers Med. 2024 Oct 10;14(10):1048. doi: 10.3390/jpm14101048. J Pers Med. 2024. PMID: 39452555 Free PMC article. Review. - Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers.
Tokat ÜM, Adibi A, Aydın E, Özgü E, Bilgiç ŞN, Tutar O, Özbek Doğançay M, Demiray İ, Demiray M. Tokat ÜM, et al. Curr Oncol. 2024 Sep 29;31(10):5838-5849. doi: 10.3390/curroncol31100434. Curr Oncol. 2024. PMID: 39451738 Free PMC article. - Biomarkers in head and neck squamous cell carcinoma: unraveling the path to precision immunotherapy.
Saini KS, Somara S, Ko HC, Thatai P, Quintana A, Wallen ZD, Green MF, Mehrotra R, McGuigan S, Pang L, Das S, Yadav K, Neric D, Cantini L, Joshi C, Iwamoto K, Dubbewar S, Vidal L, Chico I, Severson E, Lorini L, Badve S, Bossi P. Saini KS, et al. Front Oncol. 2024 Oct 8;14:1473706. doi: 10.3389/fonc.2024.1473706. eCollection 2024. Front Oncol. 2024. PMID: 39439946 Free PMC article. Review. - Low PD-L1 expression, MAP2K2 alterations, and enriched HPV gene signatures characterize brain metastases in head and neck squamous cell carcinoma.
Dennis MJ, Pavlick DC, Kacew A, Wotman M, MacConaill LE, Jones SM, Pfaff KL, Rodig SJ, Eacker S, Malig M, Reister E, Piccioni D, Kesari S, Sehgal K, Haddad RI, Cohen E, Posner MR, Deichaite I, Hanna GJ. Dennis MJ, et al. J Transl Med. 2024 Oct 22;22(1):960. doi: 10.1186/s12967-024-05761-z. J Transl Med. 2024. PMID: 39438862 Free PMC article. - Cancer cell-specific PD-L1 expression is a predictor of poor outcome in patients with locally advanced oral cavity squamous cell carcinoma.
Wang M, Qin L, Thia K, Nguyen T, MacDonald S, Belobrov S, Kranz S, Goode D, Trapani JA, Wiesenfeld D, Neeson PJ. Wang M, et al. J Immunother Cancer. 2024 Oct 2;12(10):e009617. doi: 10.1136/jitc-2024-009617. J Immunother Cancer. 2024. PMID: 39357980 Free PMC article.
References
- Chow LQM, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838–3845. doi: 10.1200/JCO.2016.68.1478. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous