Revisiting the Role of Clathrin-Mediated Endoytosis in Synaptic Vesicle Recycling - PubMed (original) (raw)
Review
Revisiting the Role of Clathrin-Mediated Endoytosis in Synaptic Vesicle Recycling
Ira Milosevic. Front Cell Neurosci. 2018.
Abstract
Without robust mechanisms to efficiently form new synaptic vesicles (SVs), the tens to hundreds of SVs typically present at the neuronal synapse would be rapidly used up, even at modest levels of neuronal activity. SV recycling is thus critical for synaptic physiology and proper function of sensory and nervous systems. Yet, more than four decades after it was originally proposed that the SVs are formed and recycled locally at the presynaptic terminals, the mechanisms of endocytic processes at the synapse are heavily debated. Clathrin-mediated endocytosis, a type of endocytosis that capitalizes on the clathrin coat, a number of adaptor and accessory proteins, and the GTPase dynamin, is well understood, while the contributions of clathrin-independent fast endocytosis, kiss-and-run, bulk endocytosis and ultrafast endocytosis are still being evaluated. This review article revisits and summarizes the current knowledge on the SV reformation with a focus on clathrin-mediated endocytosis, and it discusses the modes of SV formation from endosome-like structures at the synapse. Given the importance of this topic, future advances in this active field are expected to contribute to better comprehension of neurotransmission, and to have general implications for neuroscience and medicine.
Keywords: clathrin; dyanmin; endocytosis; endophilin; endosomes; fast endocytosis; synaptic transmission; synaptic vesicle size.
Figures
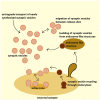
Figure 1
Schematic representation of the synaptic vesicle (SV) dynamics at the presynaptic terminal. SVs that accumulate at the presynaptic terminal originate from various sources: they are either actively transported to the synapses or recycled locally, either in the close proximity to the active zone (periactive zone) or budding from endosome-like structures. Furthermore, significant exchange of SVs happens between adjacent synapses. Active zone is depicted in dark yellow, SVs in orange, clathrin coated vesicles (CCVs) in dark brown and endosome-like structure in light-brown color.
Figure 2
Model of clathrin-mediated endocytosis, a form of endocytosis that builds on the clathrin coat, the GTPase dynamin, and a variety of accessory factors, like clathrin adaptors, BAR proteins (e.g., endophilin) and lipid phosphatases (e.g., synaptojanin-1).
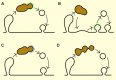
Figure 3
Modes of SV recovery from endosome-like structures. (A) Clathrin-mediated budding. (B) Fusion of endosome-like structures with the plasma membrane (a sort of homotypic fusion of bulk internalized plasma membrane fragments) followed by subsequent recovery of SV proteins from the plasma membrane by clathrin-mediated endocytosis. (C) Budding by clathrin-independent mechanisms but mediated by some coat proteins. (D) Tubulation followed by tubule fragmentation without classic coat proteins. Endosome-like structures are depicted in brown and clathin coat in green. Black lines outline the membrane contours, and blue dotted line represents clathrin-independent coat.
Similar articles
- Endophilin A and B Join Forces With Clathrin to Mediate Synaptic Vesicle Recycling in Caenorhabditis elegans.
Yu SC, Jánosi B, Liewald JF, Wabnig S, Gottschalk A. Yu SC, et al. Front Mol Neurosci. 2018 Jun 14;11:196. doi: 10.3389/fnmol.2018.00196. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29962934 Free PMC article. - Synaptic Vesicle Endocytosis Occurs on Multiple Timescales and Is Mediated by Formin-Dependent Actin Assembly.
Soykan T, Kaempf N, Sakaba T, Vollweiter D, Goerdeler F, Puchkov D, Kononenko NL, Haucke V. Soykan T, et al. Neuron. 2017 Feb 22;93(4):854-866.e4. doi: 10.1016/j.neuron.2017.02.011. Neuron. 2017. PMID: 28231467 - A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis.
Wu Y, O'Toole ET, Girard M, Ritter B, Messa M, Liu X, McPherson PS, Ferguson SM, De Camilli P. Wu Y, et al. Elife. 2014 Jun 24;3:e01621. doi: 10.7554/eLife.01621. Elife. 2014. PMID: 24963135 Free PMC article. - The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms.
Chanaday NL, Cousin MA, Milosevic I, Watanabe S, Morgan JR. Chanaday NL, et al. J Neurosci. 2019 Oct 16;39(42):8209-8216. doi: 10.1523/JNEUROSCI.1158-19.2019. J Neurosci. 2019. PMID: 31619489 Free PMC article. Review. - Modes and mechanisms of synaptic vesicle recycling.
Soykan T, Maritzen T, Haucke V. Soykan T, et al. Curr Opin Neurobiol. 2016 Aug;39:17-23. doi: 10.1016/j.conb.2016.03.005. Epub 2016 Mar 24. Curr Opin Neurobiol. 2016. PMID: 27016897 Review.
Cited by
- Computational analysis of functional SNPs in Alzheimer's disease-associated endocytosis genes.
Tey HJ, Ng CH. Tey HJ, et al. PeerJ. 2019 Sep 30;7:e7667. doi: 10.7717/peerj.7667. eCollection 2019. PeerJ. 2019. PMID: 31592138 Free PMC article. - The emerging roles of vacuolar-type ATPase-dependent Lysosomal acidification in neurodegenerative diseases.
Song Q, Meng B, Xu H, Mao Z. Song Q, et al. Transl Neurodegener. 2020 May 11;9(1):17. doi: 10.1186/s40035-020-00196-0. Transl Neurodegener. 2020. PMID: 32393395 Free PMC article. Review. - Stimulation-induced differential redistributions of clathrin and clathrin-coated vesicles in axons compared to soma/dendrites.
Tao-Cheng JH. Tao-Cheng JH. Mol Brain. 2020 Oct 16;13(1):141. doi: 10.1186/s13041-020-00683-5. Mol Brain. 2020. PMID: 33066817 Free PMC article. - The recurrent pathogenic Pro890Leu substitution in CLTC causes a generalized defect in synaptic transmission in Caenorhabditis elegans.
Pannone L, Muto V, Nardecchia F, Di Rocco M, Marchei E, Tosato F, Petrini S, Onorato G, Lanza E, Bertuccini L, Manti F, Folli V, Galosi S, Di Schiavi E, Leuzzi V, Tartaglia M, Martinelli S. Pannone L, et al. Front Mol Neurosci. 2023 May 31;16:1170061. doi: 10.3389/fnmol.2023.1170061. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37324589 Free PMC article. - Neuronal Signaling Involved in Neuronal Polarization and Growth: Lipid Rafts and Phosphorylation.
Igarashi M, Honda A, Kawasaki A, Nozumi M. Igarashi M, et al. Front Mol Neurosci. 2020 Aug 14;13:150. doi: 10.3389/fnmol.2020.00150. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32922262 Free PMC article.
References
- Blondeau F., Ritter B., Allaire P. D., Wasiak S., Girard M., Hussain N. K., et al. . (2004). Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. U S A 101, 3833–3838. 10.1073/pnas.0308186101 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources