A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy - PubMed (original) (raw)
. 2018 Apr;28(4):416-432.
doi: 10.1038/s41422-018-0011-0. Epub 2018 Feb 22.
Fei Tang 1, Mingyue Liu 1, Juanjuan Su 1, Yan Zhang 1, Wei Wu 1, Martin Devenport 2, Christopher A Lazarski 1, Peng Zhang 1, Xu Wang 1, Peiying Ye 1, Changyu Wang 3, Eugene Hwang 1, Tinghui Zhu 4, Ting Xu 4, Pan Zheng 5 6, Yang Liu 1 2
Affiliations
- PMID: 29472691
- PMCID: PMC5939050
- DOI: 10.1038/s41422-018-0011-0
A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy
Xuexiang Du et al. Cell Res. 2018 Apr.
Abstract
It is assumed that anti-CTLA-4 antibodies cause tumor rejection by blocking negative signaling from B7-CTLA-4 interactions. Surprisingly, at concentrations considerably higher than plasma levels achieved by clinically effective dosing, the anti-CTLA-4 antibody Ipilimumab blocks neither B7 trans-endocytosis by CTLA-4 nor CTLA-4 binding to immobilized or cell-associated B7. Consequently, Ipilimumab does not increase B7 on dendritic cells (DCs) from either CTLA4 gene humanized (Ctla4 h/h ) or human CD34+ stem cell-reconstituted NSG™ mice. In Ctla4 h/m mice expressing both human and mouse CTLA4 genes, anti-CTLA-4 antibodies that bind to human but not mouse CTLA-4 efficiently induce Treg depletion and Fc receptor-dependent tumor rejection. The blocking antibody L3D10 is comparable to the non-blocking Ipilimumab in causing tumor rejection. Remarkably, L3D10 progenies that lose blocking activity during humanization remain fully competent in inducing Treg depletion and tumor rejection. Anti-B7 antibodies that effectively block CD4 T cell activation and de novo CD8 T cell priming in lymphoid organs do not negatively affect the immunotherapeutic effect of Ipilimumab. Thus, clinically effective anti-CTLA-4 mAb causes tumor rejection by mechanisms that are independent of checkpoint blockade but dependent on the host Fc receptor. Our data call for a reappraisal of the CTLA-4 checkpoint blockade hypothesis and provide new insights for the next generation of safe and effective anti-CTLA-4 mAbs.
Conflict of interest statement
Y.L. and P.Z. are co-founders of, and have equity interests in OncoImmune, Inc.. M.D. is an employee of OncoImmune, Inc. and has an equity interest. The remaining authors declare no conflict of interest.
Figures
Fig. 1
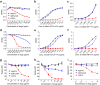
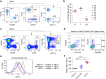
Ipilimumab exhibits poor blocking activity for B7-1-CTLA-4 and B7-2-CTLA-4 interactions if the B7-1 or B7-2 are immobilized. a–c Blocking activities of anti-human CTLA-4 mAbs Ipilimumab and L3D10 in B7-1-CTLA-4 interaction. a hB7-1-Fc was immobilized at the concentration of 0.5 μg/ml. Biotinylated CTLA-4-Fc was added at 0.25 μg/ml along with given doses of antibodies. b As in a, except that varying doses of biotinylated CTLA-4-Fc was used in the presence of a saturating dose of Ipilimumab or L3D10 (100 μg/ml). c As in a, except that varying doses of B7-1-Fc was used to coat plate and that a saturating dose of Ipilimumab or L3D10 (100 μg/ml) was used to block CTLA-4-B7-1 interaction. d–f Blocking activities of anti-human CTLA-4 mAbs Ipilimumab and L3D10 in B7-2-CTLA-4 interaction. d As in a, except that hB7-2-Fc was immobilized. e As in b, except that hB7-2-Fc was immobilized. f As in c, except that hB7-2-Fc was immobilized. Data shown in a–f are means of duplicate or triplicate optical density at 450 nm. g Blocking of CTLA-4 interaction with cell surface hB7-1. CHO cells expressing hB7-1 were incubated with biotinylated CTLA-4-Fc along with given doses of antibodies. The amounts of B7-bound CTLA-4-Fc were detected with PE-streptavidin, and mean fluorescence intensity (MFI) of PE was calculated. h Blocking of CTLA-4 interaction with cell surface mB7-2. As in g, except CHO cell-expressing mB7-2 was used. i Blocking of CTLA-4-Fc binding to spleen DCs matured with overnight LPS stimulation. As in g and h, except 0.5 μg/ml LPS-stimulated 2 × 106 splenocytes were used for each test and CD11chigh DCs (as Fig. 5b) were gated for analyzing PE intensity. Data (mean ± S.D.) shown are normalized MFI values of triplicate samples. Data shown in this figure have been repeated 2–5 times
Fig. 2
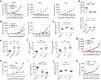
Reconciling the differential blocking effects of Ipilimumab. a–d Ipilimumab does not break up pre-formed B7-CTLA-4 complex. a, b Impact of anti-CTLA-4 mAbs on B7-complexed CTLA-4. The B7-CTLA-4 complexes were formed by adding biotinylated CTLA-4 to plates pre-coated with either B7-1 (a) or B7-2 (b). Graded doses of anti-CTLA-4 mAbs were added to plates with pre-existing B7-1-CTLA-4 complex (a) or B7-2-CTLA-4 complex (b). After 2 h, the unbound proteins were washed away and the amounts of B7-1 or B7-2-complexed CTLA-4 were detected using HRP-labeled Streptavidin. c Dissociation kinetics of B7 and CTLA-4 complex based on flow cytometric assays using B7-expressing CHO cells. Surface hB7-1 or mB7-2-expressing CHO cells (1 × 105/test) were incubated with soluble biotinylated CTLA-4-Fc (200 ng/test) for 30 min at room temperature. After washing, cells were incubated in 100 μl DPBS buffer for the indicated minutes. The amounts of B7-bound CTLA-4-Fc were detected with PE-streptavidin by flow cytometry, and the mean fluorescence intensity (MFI) of PE was calculated from triplicated samples. Data shown are results from one of two independent experiments. d L3D10 but not Ipilimumab significantly disrupts the pre-established interaction between soluble CTLA-4 and hB7-1 expressed on CHO cells. Surface hB7-1-expressing CHO cells (1 × 105/test) were incubated with soluble biotinylated CTLA-4-Fc (200 ng/test) for 30 min at room temperature. After washing, cells were incubated with given doses of antibodies in 100 μl DPBS buffer for 1 h. The amounts of B7-bound CTLA-4-Fc were detected with PE-streptavidin, and MFI of PE was calculated. The results represent one of three independent assays with similar patterns. e Ipilimumab does not relieve CTLA-4-Fc-mediated inhibition of CD28-Fc binding to B7-1-transfected J558 cells (J558-B7). J558-B7 cells were incubated with biotinylated CD28-Fc (20 μg/ml) in the presence of CTLA-4-Fc (5 μg/ml) and graded doses of anti-CTLA-4 mAbs or control IgG-Fc. Data shown are means and S.E.M. of MFI from triplicate samples and are representative of at least three independent experiments with similar results. f Kinetics of B7-1-CTLA-4 interaction when B7-1 was immobilized. g Kinetics of B7-1-CTLA-4 interaction when CTLA-4 is immobilized. Data shown in this figure have been repeated two to five times
Fig. 3
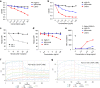
Ipilimumab is ineffective in blocking B7/CTLA-4-mediated cell–cell interactions. a Profiles of B7-1-GFP or B7-2-GFP-transfected CHO cells or CTLA-4Y201V-transfected 293T cells or mixture of B7-2 and CTLA-4 transfectants without co-incubation. b SDS-PAGE analysis for purify of Fabs used for the study. c, d Representative FACS profiles (c, Fabs used at 10 μg/ml) and dose responses (d) showing comparable binding by L3D10 and Ipilimumab Fabs to CTLA-4-OFP-transfected CHO cells. Alex Fluor 488-conjugated goat anti-human IgG (H+L) was used as the secondary antibody for the binding assay. Dose responses show similar binding activity of Ipilimumab and L3D10 Fabs. AF488-MFI, mean fluorescence intensity of Alex Fluor 488 dye. e Inhibition of B7-1-CTLA-4Y201V-mediated cell–cell interaction by anti-CTLA-4 mAb Fabs. B7-1-GFP-transfected CHO cells and CTLA-4Y201V-transfected 293T cells were co-incubated at 4 °C for 2 h in the presence of 10 μg/ml Fab or control proteins. Data shown are representative FACS profiles. f Quantitative comparison between L3D10 and Ipilimumab for their blocking of cell–cell interaction mediated by B7-1 and CTLA-4 expressed on opposing cells. As in e, except that graded doses of antibodies were added. g Inhibition of B7-2-CTLA-4Y201V-mediated cell–cell interaction by anti-CTLA-4 mAb Fabs. As in e, except that B7-2-GFP transfectants were used. h Quantitative comparison between L3D10 and Ipilimumab for their blocking of cell–cell interaction mediated by B7-2 and CTLA-4 expressed on opposing cells. As in f, except that B7-2-GFP-transfected CHO cells were used. All assays have been repeated at least two times
Fig. 4
Ipilimumab is ineffective in blocking B7-trans-endocytosis by CTLA-4. a FACS profiles of B7-2-GFP-transfected or CTLA-4-OFP-transfected CHO cell lines used for trans-endocytodosis assay. b Rapid trans-endocytosis of B7-2 by CTLA-4. B7-2-GFP transfectants and CTLA-4-OFP transfectants were co-incubated for 0, 0.5, 1 and 4 h at 37 °C. c Lack of trans-endocytosis of B7-H2 by CTLA-4. As in b, except that B7-H2-GFP-transfected P815 cells and data at 0, 1 and 4 h of co-culturing are presented. d Representative profiles depicting differential blockade of trans-endocytosis of B7-1-GFP by CTLA-4-OFP-expressing CHO cells during coculture in the presence of control hIgG-Fc or Fab from either Ipilimumab or L3D10 (10 μg/ml) for 4 h. e Dose–response curve depicting inhibition of B7-1 trans-endocytosis by L3D10 and Ipilimumab Fab. As in d, except varying doses of control hIgG-Fc or Fab were added to the co-culturing. f As in d, except that B7-2-GFP-transfected CHO cells were used. g Dose–response curve depicting inhibition of B7-2 trans-endocytosis by L3D10 and Ipilimumab Fab. As in e, except that B7-2-GFP-transfected CHO cells were used. Data shown (mean ± S.D.) are % of trans-endocytosis over varying doses of Fab. All assays have been repeated at least three times
Fig. 5
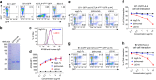
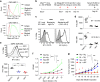
Ipilimumab does not block B7-CTLA-4 interaction in vivo. a Diagram of experimental design. b Representative data showing the phenotype of CD11b+CD11chigh DC analyzed for B7 expression. c Representative histograms depicting the levels of mB7-1 on DC from mice that received control hIgG-Fc, L3D10 or Ipilimumab. Data in the top panel show antibody effect in homozygous human CTLA4 knock-in mice (Ctla4 h/h), while that in the bottom panel show antibody effect in the heterozygous mice (Ctla4 h/m). d As in c, except that expression of mB7-2 is shown. Data shown in c and d are representative of those from three mice per group and have been repeated once. e In human CTLA4 homozygous mice, L3D10 but not Ipilimumab-induced upregulation of mB7-1 (left panel) and mB7-2 (right panel). Data shown (mean ± S.E.M.) are summarized from two experiments involving a total of six mice per group. f As in e, except that heterozygous mice are used. Neither L3D10 nor Ipilimumab blocks B7-CTLA-4 interaction in mice that co-dominantly express both mouse and human Ctla4 genes. Statistical significance was determined using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant
Fig. 6
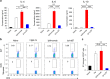
Ipilimumab does not block human B7-human CTLA-4 interaction in vivo. a FACS profiles depicting composition of human leukocytes among the peripheral blood leukocytes (PBL) of NSGTM mice reconstituted with human cord blood CD34+ cells. b Summary data of individual mice as analyzed in a. c Normal composition of Treg cells and DCs in spleen of humanized NSGTM mice. d Expression of FOXP3 and CTLA-4 among human CD4 T cells in mice spleen. e, f L3D10 but not Ipilimumab blocks human B7-2-human CTLA-4 interaction in the human cord blood CD34+ stem cell-reconstituted NSG™ mice. The humanized mice received intraperioneal treatment of either control Ig or anti-CTLA-4 mAbs (500 μg/mouse). Splenocytes were harvested at 24 h after injection and analyzed for expression of B7 on DC. e Representative profiles of hB7-2 on DC. f Summary data (mean ± S.E.M.) from two independent experiments. The mean data in the control mice are artificially defined as 100 and those in experimental groups are normalized against the control. Statistical significance was determined using Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant
Fig. 7
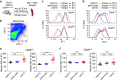
Blocking the B7-CTLA-4 interaction does not contribute to anti-CTLA-4 mAbs elicited cancer immunotherapeutic activity and intratumorial Treg depletion. a Comparable immunotherapeutic effect despite vastly different blocking activity by two anti-CTLA-4 mAbs. 5 × 105 or 1 × 106 MC38 tumor cells were injected (s.c.) into Ctla4 h/h mice (n = 5–6), and mice were treated (i.p.) with 100 μg (left), 30 μg (middle) or 10 μg (right) Ipilimumab, L3D10 or control hIgG-Fc per mouse on days 7, 10, 13 and 16, as indicated by arrows. Data represent mean ± S.E.M. of 5–6 mice per group. Statistical analyses were performed by two-way repeated measures ANOVA (treatment × time). For 100 μg treatments, Ipilimumab vs hIgG-Fc: P < 0.0001; L3D10 vs hIgG-Fc: P < 0.0001; Ipilimumab vs L3D10: P = 0.0699. For 30 μg treatments, Ipilimumab vs hIgG-Fc: P < 0.0001; L3D10 vs hIgGFc: P < 0.0001; Ipilimumab vs L3D10: P = 0.9969. For 10 μg treatments, Ipilimumab vs hIgG-Fc: P < 0.0001; L3D10 vs hIgG-Fc: P < 0.0001; Ipilimumab vs L3D10: P = 0.9988. Data are representative of 3–5 independent experiments. b Ipilimumab and L3D10 have similar therapeutic effect for B16 melanoma growth. 1 × 105 B16 tumor cells were injected (s.c.) into Ctla4 h/h mice (n = 4–5), and mice were treated (i.p.) with 100 μg (left) or 250 μg (right) Ipilimumab, L3D10 or control hIgG-Fc on day 11, 14, 17(left) or on day 2, 5 and 8 (right), as indicated by arrows. For the left panel, Ipilimumab vs hIgG-Fc: P = 0.0265; L3D10 vs hIgG-Fc: P = 0.0487; Ipilimumab vs L3D10: P = 0.302. For the right panel, Ipilimumab vs hIgG-Fc: P = 0.00616; L3D10 vs hIgG-Fc: P = 0.0269: Ipilimumab vs L3D10: P = 0.370, Data represent mean ± S.E.M. of 4–5 mice per group. c–f Blocking B7-CTLA-4 interaction does not contribute to selective depletion of Treg cells in tumor microenvironment in the Ctla4 h/h mice. L3D10 and Ipilimumab did not delete Treg cells in the spleen (c) of mice at 3 days after third treatment. Data shown are the percentage of Foxp3+ cells among CD4 T cells in Ctla4 h/h mice. n = 6 mice for each group. Both L3D10 and Ipilimumab depleted Treg cells in tumors transplanted into the Ctla4 h/h mice, as determined by % Treg cells among CD4 T cells (d, upper), absolute Treg cell number (d, lower) and CD8/Treg ratios (e). Summary data from two experiments involving seven mice per group are presented in d (upper panel) and e. The numbers of Foxp3+ cells (d, lower panel) in the tumor from Ctla4 h/h mice were counted by flow cytometry on 3 days after the third antibody treatment. n = 5 for each group. Statistical analyses were performed by ordinary one-way ANOVA with Tukey’s multiple comparisons test. f Blocking B7-CTLA-4 interaction does not contribute to increased IFNγ-producing cells among tumor-infiltrating CD4 (left) or CD8 (right) T cells. Summary data are from two experiments involving seven mice per group. Single-cell suspensions of collagenase-digested tumors were prepared between 13 or 16 days and cultured in the presence of Golgi blocker for 4 h and stained for intracellular cytokines. g–j In Ctla4 h/m mice where neither antibody blocks the B7-CTLA-4 interaction, both L3D10 and Ipilimumab induce robust tumor rejection and intratumorial Treg depletion. As in a, except that heterozygous mice that express both mouse and human CTLA-4 were used. g, h Both higher doses (g, 100 μg/mouse/ injection) and lower doses (h, 10 μg/mouse/injection) of antibody treatments showed effective therapeutically effects. In g, Ipilimumab vs hIgG-Fc: P < 0.0001; L3D10 vs hIgG-Fc: P < 0.0001; Ipilimumab vs L3D10: P = 0.4970. Data are representative of five independent experiments. Treg cells were selectively depleted in the tumor (i) but not in the spleen (j) of Ctla4 h/m mice that neither antibodies significantly blocked B7-CTLA-4 interaction in vivo. Data (mean ± S.E.M.) shown in c, d, e and i are the percentage of Treg cells at 18 (experiment 1) or 20 days (experiment 2) after tumor cell challenge and 11 or 13 days after initiation of 3 or 4 anti-CTLA-4 mAb treatments as indicated in arrows. Statistical significance in c–f and i, j was determined using Mann–Whitney test. k Anti-FcR mAb administration abrogated the therapeutic effect of Ipilimumab. 5 × 105 MC38 tumor cells were injected (s.c.) into Ctla4 h/h mice, and mice were treated (i.p.) with 30 μg Ipilimumab alone, or 30 μg Ipilimumab (black arrow) plus 1 mg 2.4G2 (red arrow) or control hIgG-Fc on days 7, 10, 13 and 16, as indicated. Statistical analyses were performed by two-way repeated measures ANOVA (treatment × time). Ipilimumab vs hIgG-Fc: P = 0.0003; Ipilimumab plus 2.4G2 vs hIgG-Fc: P = 0.6962; Ipilimumab plus 2.4G2 vs Ipilimumab: P = 0.0259
Fig. 8
Humanized L3D10 progenies (HL12 and HL32) that lost blocking activities remain effective in local Treg depletion and tumor rejection. a Binding activities of HL12, HL32 and L3D10 to 1 μg/ml immobilized polyhistidine-tagged CTLA-4. b HL12 and HL32 failed to block the B7-1-CTLA-4 interaction. B7-1-Fc was immobilized at a concentration of 0.5 μg/ml. Biotinylated CTLA-4-Fc was added at 0.25 μg/ml along with grading concentration of anti-CTLA-4 mAbs. c HL12 and HL32 barely block the B7-2-CTLA-4 interaction. As in b, except B7-2-Fc is immobilized. d HL12 and HL32 failed to upregulates B7-1 and B7-2 in vivo. As in Fig. 5, Ctla4 h/h mice received 500 μg/ mouse/injection of control hIgG-Fc or anti-CTLA-4 mAbs. Spleen cells were collected next day to determine levels of B7-1 and B7-2 on CD11b+CD11chigh DCs, as detailed in Fig. 5. n = 3 for each group. e–g Similar as L3D10, HL12 and HL32 showed selective depletion of Treg cells in the tumor microenvironment in the Ctla4 h/h mice. As in Fig. 7, L3D10, HL12 and HL32 elicited comparable and efficient depletion of Treg cells in tumor (e), but did not deplete Treg cells in spleen (f) and tumor-draining lymph node (g). Data shown were pooled from two experiments. n = 5 mice for each group. Mice were killed 1 day after one injection of 100 μg indicated drug. h Efficient rejection of MC38 tumors by Ipilimumab and humanized L3D10 antibodies HL12 and HL32. Mice-bearing MC38 were treated on days 7, 10, 13 and 16 days after tumor cells inoculation with 100 μg control IgG-Fc or Ipilimumab or HL12, HL32. Data shown are means and S.E.M. of tumor volume. n = 6 mice for each group. Statistical analyses were performed by two-way repeated measures ANOVA (treatment × time). Ipilimumab vs hIgG-Fc: P = 0.034; HL12 vs hIgG-Fc: P = 0.037; HL32 vs hIgG-Fc: P = 0.0336; HL12 vs Ipilimumab : P = 0.9021; HL32 vs Ipilimumab : P = 0.9972 ; HL32 vs HL12: P = 0.7250. i HL32 and L3D10 are comparably effective in treatment of B16 tumor cells in a minimal disease model. 1 × 105 B16 tumor cells were injected (s.c.) into Ctla4 h/h mice (n = 4–5), and mice were treated (i.p.) with 250 μg of Ipilimumab, L3D10, HL32 or control IgG-Fc on days 2, 5 and 8, as indicated by arrows. HL32 vs hIgG-Fc: P = 0.0002; L3D10 vs HL32: P = 0.9998; Ipilimumab vs HL32: P = 0.8899. Data represent mean ± S.E.M. of 5–6 mice per group
Fig. 9
Therapeutic effect of Ipilumumab is not achieved by blocking CTLA-4-B7 negative signaling. a Confirmation of the blocking activities of anti-B7 mAbs. CHO cells expressing mouse B7-1 or B7-2 were incubated with a mixture of antibodies (20 μg/ml) and biotinylated human CTLA-4-Fc (2 μg/ml) for 1 h. After washing away unbound proteins, the cell surface CTLA-4-Fc was detected by PE-conjugated streptavidin and measured by flow cytometry. Data shown are representative FACS profiles and have been repeated two times. b Diagram of experimental design. MC38 tumor-bearing Ctla4 h/m mice received anti-B7-1 and anti-B7-2 antibodies (300 μg/mouse/injection, once every 3 days for a total of three injections) in conjunction with either control Ig or Ipilimumab, mice that received Ipilimumab without anti-B7-1 and anti-B7-2 were used as positive control for tumor rejection. c, d Saturation of B7-1 and B7-2 by antibody treatments as diagrammed in b. The PBL were stained with FITC-conjugated anti-B7-1 and anti-B7-2 mAbs at 24 h after the last anti-B7 treatment on day 13. PBL from _Cd80_−/−_Cd86_−/− mice were used as negative control. e Complete blocking of B7-2 in vivo. As in c and d, except that CD45+ leukocytes were gated from single-cell suspensions of draining lymph nodes in mice-bearing MC38 were used. The top panel depicts profiles of B7-2 staining, while the lower panel shows the mean fluorescence intensities. This study has been repeated three times. f Ablation of antibody responses confirmed the functional blockade of B7 by anti-B7-1 and anti-B7-2 mAbs. Sera were collected at day 22 after tumor challenge to evaluate anti-human IgG antibody response. g Saturating blocking by anti-B7-1 and anti-B7-2 mAbs does not affect immunotherapeutic effect of Ipilimumab. Data shown in g are tumor volumes over time and have been repeated twice with similar results. Data in d–g represent mean ± S.E.M.; n.s., not significant
Fig. 10
In vivo treatment of anti-B7 mAbs prevents Ipilimumab-mediated T cell activation and de novo priming of CD8 T cell. (a) Functional blockade of B7 by anti-B7-1 (1G10) and anti-B7-2 (GL1) mAbs prevented Ipilimumab-induced CD4 T cell activation. As in Fig. 9, MC38 tumor-bearing Ctla4 h/h mice (n = 5 for each group) were treated intraperitoneally with hIgG-Fc (100 μg/mouse/injection), Ipilimumab (100 μg/mouse/injection) or Ipilimumab plus anti-mB7 mAbs (300 μg 1G10 plus 300 μg GL1/mouse/injection) on day 7, 10 and 13 and killed on day 14. Sex and age-matched, tumor-free Ctla4 h/h mice were used as control naive mice. Spleen T cells from these mice were purified by MACS negative selection and co-cultured with naive spleen DCs in the presence of 10 μg/ml hIgG-Fc for 4 days. The levels of Th2 cytokines (including IL-4, IL-6 and IL-10) in the supernatant were quantitated by cytokine beads assays (CBA). b, c Anti-B7 mAbs prevented Ipilimumab-induced priming of antigen-specific CD8 T cells. As in a, except that all mice (n = 4 for each group) were immunized subcutaneously with 50 μg SIY peptide emulsified in 100 μl Complete Freund’s Adjuvant (CFA) on day 8. Mice were killed on day 15 and tumor-draining lymph nodes were collected to evaluate SIY-specific CD8 T cells (gated on CD3+ CD4− cells) by tetramer staining. OVA tetramer was used for control staining. Representative FACS profiles (b) and summary data (c) are shown. Data shown are representative of two independent experiments with similar results
Comment in
- Anti-CTLA-4 immunotherapy: uncoupling toxicity and efficacy.
Pol J, Kroemer G. Pol J, et al. Cell Res. 2018 May;28(5):501-502. doi: 10.1038/s41422-018-0031-9. Epub 2018 Mar 28. Cell Res. 2018. PMID: 29593340 Free PMC article. No abstract available.
Similar articles
- Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice.
Du X, Liu M, Su J, Zhang P, Tang F, Ye P, Devenport M, Wang X, Zhang Y, Liu Y, Zheng P. Du X, et al. Cell Res. 2018 Apr;28(4):433-447. doi: 10.1038/s41422-018-0012-z. Epub 2018 Feb 20. Cell Res. 2018. PMID: 29463898 Free PMC article. - CTLA-4 antibody ipilimumab negatively affects CD4+ T-cell responses in vitro.
Rosskopf S, Leitner J, Zlabinger GJ, Steinberger P. Rosskopf S, et al. Cancer Immunol Immunother. 2019 Aug;68(8):1359-1368. doi: 10.1007/s00262-019-02369-x. Epub 2019 Jul 22. Cancer Immunol Immunother. 2019. PMID: 31332464 Free PMC article. - Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade?
Tang F, Du X, Liu M, Zheng P, Liu Y. Tang F, et al. Cell Biosci. 2018 Apr 18;8:30. doi: 10.1186/s13578-018-0229-z. eCollection 2018. Cell Biosci. 2018. PMID: 29713453 Free PMC article. - Clinical experience with CTLA-4 blockade for cancer immunotherapy: From the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment.
Lisi L, Lacal PM, Martire M, Navarra P, Graziani G. Lisi L, et al. Pharmacol Res. 2022 Jan;175:105997. doi: 10.1016/j.phrs.2021.105997. Epub 2021 Nov 24. Pharmacol Res. 2022. PMID: 34826600 Review. - CTLA-4 and PD-1 Control of T-Cell Motility and Migration: Implications for Tumor Immunotherapy.
Brunner-Weinzierl MC, Rudd CE. Brunner-Weinzierl MC, et al. Front Immunol. 2018 Nov 27;9:2737. doi: 10.3389/fimmu.2018.02737. eCollection 2018. Front Immunol. 2018. PMID: 30542345 Free PMC article. Review.
Cited by
- Differentiation fate of a stem-like CD4 T cell controls immunity to cancer.
Cardenas MA, Prokhnevska N, Sobierajska E, Gregorova P, Medina CB, Valanparambil RM, Greenwald R, DelBalzo L, Bilen MA, Joshi SS, Narayan VM, Master VA, Sanda MG, Kissick HT. Cardenas MA, et al. Nature. 2024 Oct 23. doi: 10.1038/s41586-024-08076-7. Online ahead of print. Nature. 2024. PMID: 39443797 - Anti-CTLA-4 treatment suppresses hepatocellular carcinoma growth through Th1-mediated cell cycle arrest and apoptosis.
Morihara H, Yamada T, Tona Y, Akasaka M, Okuyama H, Chatani N, Shinonome S, Ueyama A, Kuwabara K, Fujio Y. Morihara H, et al. PLoS One. 2024 Aug 6;19(8):e0305984. doi: 10.1371/journal.pone.0305984. eCollection 2024. PLoS One. 2024. PMID: 39106430 Free PMC article. - Lipid-based nanosystems: the next generation of cancer immune therapy.
Cheng Z, Fobian SF, Gurrieri E, Amin M, D'Agostino VG, Falahati M, Zalba S, Debets R, Garrido MJ, Saeed M, Seynhaeve ALB, Balcioglu HE, Ten Hagen TLM. Cheng Z, et al. J Hematol Oncol. 2024 Jul 19;17(1):53. doi: 10.1186/s13045-024-01574-1. J Hematol Oncol. 2024. PMID: 39030582 Free PMC article. Review. - Treatment Options for Hepatocellular Carcinoma Using Immunotherapy: Present and Future.
Wei H, Dong C, Li X. Wei H, et al. J Clin Transl Hepatol. 2024 Apr 28;12(4):389-405. doi: 10.14218/JCTH.2023.00462. Epub 2024 Feb 28. J Clin Transl Hepatol. 2024. PMID: 38638377 Free PMC article. Review. - The expression of CTLA-4 in hepatic alveolar echinococcosis patients and blocking CTLA-4 to reverse T cell exhaustion in _Echinococcus multilocularis_-infected mice.
Yang Y, Wuren T, Wu B, Cheng S, Fan H. Yang Y, et al. Front Immunol. 2024 Mar 28;15:1358361. doi: 10.3389/fimmu.2024.1358361. eCollection 2024. Front Immunol. 2024. PMID: 38605966 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous