Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial - PubMed (original) (raw)
. 2018 Feb 24;8(2):e017740.
doi: 10.1136/bmjopen-2017-017740.
Lorenz Uhlmann 2, Walter E Haefeli 3, Justine Rochon 2, Marjan van den Akker 4 5, Rafael Perera 6, Corina Güthlin 1, Martin Beyer 1, Frank Oswald 7, Jose Maria Valderas 8, J André Knottnerus 4, Ferdinand M Gerlach 1, Sebastian Harder 9
Affiliations
- PMID: 29478012
- PMCID: PMC5855483
- DOI: 10.1136/bmjopen-2017-017740
Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial
Christiane Muth et al. BMJ Open. 2018.
Abstract
Objectives: Investigate the effectiveness of a complex intervention aimed at improving the appropriateness of medication in older patients with multimorbidity in general practice.
Design: Pragmatic, cluster randomised controlled trial with general practice as unit of randomisation.
Setting: 72 general practices in Hesse, Germany.
Participants: 505 randomly sampled, cognitively intact patients (≥60 years, ≥3 chronic conditions under pharmacological treatment, ≥5 long-term drug prescriptions with systemic effects); 465 patients and 71 practices completed the study.
Interventions: Intervention group (IG): The healthcare assistant conducted a checklist-based interview with patients on medication-related problems and reconciled their medications. Assisted by a computerised decision support system, the general practitioner optimised medication, discussed it with patients and adjusted it accordingly. The control group (CG) continued with usual care.
Outcome measures: The primary outcome was a modified Medication Appropriateness Index (MAI, excluding item 10 on cost-effectiveness), assessed in blinded medication reviews and calculated as the difference between baseline and after 6 months; secondary outcomes after 6 and 9 months' follow-up: quality of life, functioning, medication adherence, and so on.
Results: At baseline, a high proportion of patients had appropriate to mildly inappropriate prescriptions (MAI 0-5 points: n=350 patients). Randomisation revealed balanced groups (IG: 36 practices/252 patients; CG: 36/253). Intervention had no significant effect on primary outcome: mean MAI sum scores decreased by 0.3 points in IG and 0.8 points in CG, resulting in a non-significant adjusted mean difference of 0.7 (95% CI -0.2 to 1.6) points in favour of CG. Secondary outcomes showed non-significant changes (quality of life slightly improved in IG but continued to decline in CG) or remained stable (functioning, medication adherence).
Conclusions: The intervention had no significant effects. Many patients already received appropriate prescriptions and enjoyed good quality of life and functional status. We can therefore conclude that in our study, there was not enough scope for improvement.
Trial registration number: ISRCTN99526053. NCT01171339; Results.
Keywords: Medication Appropriateness Index; computer-assisted drug therapy; medication reconciliation; multimorbidity; multiple chronic conditions; polypharmacy.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: CM, FMG, CG, MB, SH, WEH, JR and LU report receiving grants from the German Federal Ministry of Education and Research, BMBF, grant number 01GK0702, during the course of the study. WEH reports other grants from Dosing, Heidelberg, during the course of the study; he received personal fees, non-financial support and other from Aqua Institute Göttingen; personal fees, non-financial support and other from Aspen Europe; personal fees and other from Diaplan; grants, personal fees, non-financial support and other from Actelion; personal fees and other from GSK GER/UK/Slovakia/France; personal fees from Thieme Verlag; personal fees and other from Daiichi Sankyo; personal fees and other from Bristol-Myers Squibb; personal fees and other from MSD Sharp & Dohme; personal fees and other from AstraZenica; personal fees and other from Boehringer; personal fees and other from Grünenthal; personal fees and other from KWHC; personal fees and other from Novartis; personal fees and other from Berlin-Chemie; grants, personal fees and other from Landesapothekerkammer BW; grants and other from BMBF (DZIF, ESTHER); grants, personal fees, non-financial support and other from BayerPharma; grants from CHIESI; personal fees and other from Doctrina Med; personal fees and other from GSK France, UK, Germany, Slovakai; personal fees, non-financial support and other from Pfizer; grants from Smooth ClinicalTrials; grants from Sumaya Biotec; grants from Klaus Tschira Stiftung; other from University Frankfurt; grants from Vaximm, outside the submitted work. FO, MvdA, JMV, RP and JAK have nothing to disclose.
Figures
Figure 1
PaT Plot of the Prioritising Multimedication in Multimorbidity (PRIMUM) trial. †Structured symptoms of side effects: dizziness, dyspnoea, tachycardia/palpitations, nausea/vomiting, abdominal pain, bleeding diathesis, difficulties urinating, ankle oedema—frequency expressed as occurrence on 1 day/several days/almost every day during the past 2 weeks. CDSS, computerised decision support system.
Figure 2
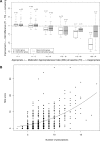
Distribution and changes in the MAI using baseline values and number of prescriptions. (A) Changes in MAI scores in intervention and control groups 6 months after baseline compared with baseline values (absolute numbers of study participants and boxes and whiskers per subgroup are provided). (B) MAI scores at baseline in terms of the number of prescriptions (higher diameters of drops represent higher numbers of study participants).
Figure 3
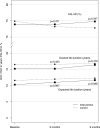
Secondary outcomes related to patients’ self-reported quality of life measures. DLD, desired life duration; ELD, expected life duration; EQ-5D, EuroQol five dimensions.
Similar articles
- Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot).
Muth C, Harder S, Uhlmann L, Rochon J, Fullerton B, Güthlin C, Erler A, Beyer M, van den Akker M, Perera R, Knottnerus A, Valderas JM, Gerlach FM, Haefeli WE. Muth C, et al. BMJ Open. 2016 Jul 25;6(7):e011613. doi: 10.1136/bmjopen-2016-011613. BMJ Open. 2016. PMID: 27456328 Free PMC article. Clinical Trial. - Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (Multi-PAP project).
Prados-Torres A, Del Cura-González I, Prados-Torres D, López-Rodríguez JA, Leiva-Fernández F, Calderón-Larrañaga A, López-Verde F, Gimeno-Feliu LA, Escortell-Mayor E, Pico-Soler V, Sanz-Cuesta T, Bujalance-Zafra MJ, Morey-Montalvo M, Boxó-Cifuentes JR, Poblador-Plou B, Fernández-Arquero JM, González-Rubio F, Ramiro-González MD, Coscollar-Santaliestra C, Martín-Fernández J, Barnestein-Fonseca MP, Valderas-Martínez JM, Marengoni A, Muth C; Multi-PAP Group. Prados-Torres A, et al. Implement Sci. 2017 Apr 27;12(1):54. doi: 10.1186/s13012-017-0584-x. Implement Sci. 2017. PMID: 28449721 Free PMC article. Clinical Trial. - Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot.
McCarthy C, Clyne B, Corrigan D, Boland F, Wallace E, Moriarty F, Fahey T, Hughes C, Gillespie P, Smith SM. McCarthy C, et al. Implement Sci. 2017 Aug 1;12(1):99. doi: 10.1186/s13012-017-0629-1. Implement Sci. 2017. PMID: 28764753 Free PMC article. Clinical Trial. - Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial.
Mahlknecht A, Wiedermann CJ, Sandri M, Engl A, Valentini M, Vögele A, Schmid S, Deflorian F, Montalbano C, Koper D, Bellmann R, Sönnichsen A, Piccoliori G. Mahlknecht A, et al. BMC Geriatr. 2021 Nov 23;21(1):659. doi: 10.1186/s12877-021-02612-0. BMC Geriatr. 2021. PMID: 34814835 Free PMC article. Review. - Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus.
Muth C, Blom JW, Smith SM, Johnell K, Gonzalez-Gonzalez AI, Nguyen TS, Brueckle MS, Cesari M, Tinetti ME, Valderas JM. Muth C, et al. J Intern Med. 2019 Mar;285(3):272-288. doi: 10.1111/joim.12842. Epub 2018 Dec 10. J Intern Med. 2019. PMID: 30357955
Cited by
- How to Improve Healthcare for Patients with Multimorbidity and Polypharmacy in Primary Care: A Pragmatic Cluster-Randomized Clinical Trial of the MULTIPAP Intervention.
Del Cura-González I, López-Rodríguez JA, Leiva-Fernández F, Gimeno-Miguel A, Poblador-Plou B, López-Verde F, Lozano-Hernández C, Pico-Soler V, Bujalance-Zafra MJ, Gimeno-Feliu LA, Aza-Pascual-Salcedo M, Rogero-Blanco M, González-Rubio F, García-de-Blas F, Polentinos-Castro E, Sanz-Cuesta T, Castillo-Jimena M, Alonso-García M, Calderón-Larrañaga A, Valderas JM, Marengoni A, Muth C, Prados-Torres JD, Prados-Torres A; Multi-Pap Group. Del Cura-González I, et al. J Pers Med. 2022 May 6;12(5):752. doi: 10.3390/jpm12050752. J Pers Med. 2022. PMID: 35629175 Free PMC article. - Implementation and evaluation of a complex intervention to improve information availability at the interface between inpatient and outpatient care in older patients with multimorbidity and polypharmacy (HYPERION-TransCare) - study protocol for a pilot and feasibility cluster-randomized controlled trial in general practice in Germany.
Klein AA, Petermann J, Brosse F, Piller S, Kramer M, Hanf M, Dinh TS, Schulz-Rothe S, Engler J, Mergenthal K, Seidling HM, Klasing S, Timmesfeld N, van den Akker M, Voigt K. Klein AA, et al. Pilot Feasibility Stud. 2023 Aug 22;9(1):146. doi: 10.1186/s40814-023-01375-2. Pilot Feasibility Stud. 2023. PMID: 37608345 Free PMC article. - Preliminary feasibility assessment of a targeted, pharmacist-led intervention for older adults with polypharmacy: a mixed-methods study.
Liu L, Brokenshire B, Davies D, Harrison J. Liu L, et al. Int J Clin Pharm. 2024 Oct;46(5):1102-1113. doi: 10.1007/s11096-024-01740-y. Epub 2024 May 16. Int J Clin Pharm. 2024. PMID: 38753077 Free PMC article. - A narrative review of evidence to guide deprescribing among older adults.
Ie K, Aoshima S, Yabuki T, Albert SM. Ie K, et al. J Gen Fam Med. 2021 May 28;22(4):182-196. doi: 10.1002/jgf2.464. eCollection 2021 Jul. J Gen Fam Med. 2021. PMID: 34221792 Free PMC article. Review. - Decision support software-guided medication reviews in elderly patients with polypharmacy: a prospective analysis of routine data from community pharmacies (OPtiMed study protocol).
Maierhöfer S, Waltering I, Jacobs M, Würthwein G, Appelrath M, Koling S, Hempel G. Maierhöfer S, et al. J Pharm Policy Pract. 2022 Dec 9;15(1):100. doi: 10.1186/s40545-022-00495-z. J Pharm Policy Pract. 2022. PMID: 36494764 Free PMC article.
References
- van den Akker M, Buntinx F, Knottnerus J. Comorbidity or multimorbidity: what’s in a name. A review of literature. Eur J Gen Pract 1996;2:65–70. 10.3109/13814789609162146 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical