Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere - PubMed (original) (raw)
doi: 10.1038/s41467-018-03168-1.
Lorena Silva 1 2, Ludmila Santos Silva 1 2, Jacques Yaacoub Bou Khalil 3, Rodrigo Rodrigues 2, Thalita Arantes 2, Felipe Assis 2, Paulo Boratto 2, Miguel Andrade 4, Erna Geessien Kroon 2, Bergmann Ribeiro 4, Ivan Bergier 5, Herve Seligmann 1, Eric Ghigo 1, Philippe Colson 1, Anthony Levasseur 1, Guido Kroemer 6 7 8 9 10 11 12, Didier Raoult 13, Bernard La Scola 14
Affiliations
- PMID: 29487281
- PMCID: PMC5829246
- DOI: 10.1038/s41467-018-03168-1
Tailed giant Tupanvirus possesses the most complete translational apparatus of the known virosphere
Jônatas Abrahão et al. Nat Commun. 2018.
Abstract
Here we report the discovery of two Tupanvirus strains, the longest tailed Mimiviridae members isolated in amoebae. Their genomes are 1.44-1.51 Mb linear double-strand DNA coding for 1276-1425 predicted proteins. Tupanviruses share the same ancestors with mimivirus lineages and these giant viruses present the largest translational apparatus within the known virosphere, with up to 70 tRNA, 20 aaRS, 11 factors for all translation steps, and factors related to tRNA/mRNA maturation and ribosome protein modification. Moreover, two sequences with significant similarity to intronic regions of 18 S rRNA genes are encoded by the tupanviruses and highly expressed. In this translation-associated gene set, only the ribosome is lacking. At high multiplicity of infections, tupanvirus is also cytotoxic and causes a severe shutdown of ribosomal RNA and a progressive degradation of the nucleus in host and non-host cells. The analysis of tupanviruses constitutes a new step toward understanding the evolution of giant viruses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
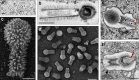
Tupanvirus soda lake particles and cycle. a Optical microscopy of Tupanvirus particles after haemacolour staining (1000 × ). Scale bar, 2 µm. b Super particle (>1000 nm) observed by transmission electron microscopy (TEM). Scale bar, 500 nm. c, d Scanning electron microscopy (SEM) of Tupanvirus particles. Scale bars 250 nm and 1 µm, respectively. e, f The initial steps of infection in A. castellanii involve the release of both capsid (e) and tail (f) content into the amoeba cytoplasm (red arrows). Scale bars, 350 nm and 450 nm, respectively
Fig. 2
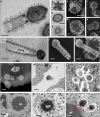
Tupanvirus soda lake particles and cycle features. a Transmission electron microscopy (TEM) highlights the inner elements of the whole particle. Scale bar, 200 nm. b Star-gate vertex transversally cut. Scale bar, 100 nm. c Capsid transversally cut. Scale bar, 100 nm. d Tail transversally cut. Scale bar, 200 nm. e–h Scanning electron microscopy (SEM) of purified particles. Scale bars, 250 nm. The treatment of particles with lysozyme, bromelain and proteinase-K removed most of the fibers, revealing head and tail junction details. Super particles (>2000 nm) could be observed by TEM (i) and SEM (j, k). Scale bars, 400 nm. Cycle steps are shown from l–r. l Viral particle attachment to Acanthamoeba castellanii surface; scale bar, 500 nm; m phagocytosis; scale bar, 500 nm; n particles in a phagosome; scale bar, 500 nm; o early viral factory; scale bar, 500 nm; and p, q mature viral factories. Scale bars 1 µm and 250 nm, respectively. Arrows highlight tail formation associated with the viral factories. VF viral factory
Fig. 3
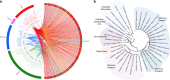
Tupanvirus soda lake rhizome and Mimiviridae family B DNA polymerase tree. a The rhizome shows that most Tupanvirus soda lake genes have mimiviruses as best hits. However, correspondence among Tupanvirus and Archaea, Eukaryota, Bacteria and other viruses was also observed. b Family B DNA polymerase maximum likelihood phylogenetic tree demonstrating the position of Tupanvirus among Mimiviridae members, likely forming a new genus
Fig. 4
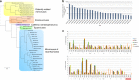
Tupanvirus soda lake hierarchical clustering, promoter’s motifs and amino-acid usage analysis. a Hierarchical clustering tree based on presence-absence matrix of cluster of orthologous genes shared by Mimiviridae members. b Frequency of mimivirus AAAATTGA canonical promoter motifs in Tupanvirus intergenic regions. We also analyzed the presence of the AAAATTGA motif with SNPs, considering each motif position. c Comparative amino-acid usage analysis of Tupan, A. castellanii and mimivirus lineages a, b and c. The amino-acid usage for protein sequences was calculated using the CGUA (General Codon Usage Analysis) tool
Fig. 5
Tupanvirus genome-translation-related factors. a Circular representation of Tupanvirus soda lake genome highlighting its translation-related factors (aaRS, tRNAs and PSF). The box (upright) summarizes this information and considers the Tupanvirus deep ocean data set. b Network of shared categories of translation-related genes (not considering ribosomal proteins) present in tupanviruses, Mimivirus (APMV), Klosneuvirus, Catovirus, Hokovirus, Indivirus and cellular world organism—Encephalitozoon cuniculi (Eukaryota), Nanoarchaeum equitans (Archaea) and Candidatus Carsonella ruddii (Bacteria). The diameter of the organism’s circles (numbers) is proportional to the number of translation-related genes present in those genomes. CDS coding sequences, tRNA transfer RNA, aaRS aminoacyl tRNA synthetase, PSF protein synthesis factors
Fig. 6
Adjacent regions of 18S rRNA intronic sequences in the genus Mimivirus and Tupanvirus and maximum likelihood phylogenetic tree of 18S rRNA intronic region. Core sequences are represented for lineages A (a), B (b), C (c), Tupanvirus soda lake (d) and Tupanvirus deep ocean (e). Phylogenetic tree of 18S rRNA intronic region present in mimivirus (e), Phycodnaviridae, eukaryotes and fungi mitochondrion
Fig. 7
Tupanvirus soda lake biological features in a host (A. castellanii) and non-host (Tetrahymena sp.). a Expression of Tupanvirus 18S intronic sequence-copy 1 transcript, 12 h post-infection observed by fluorescence in situ hybridization (FISH) (red). Tupanvirus-induced shutdown of A. castellanii ribosomal RNA18S transcripts (green). Scale bars, 10 µm. b Tupanvirus, but not mimivirus, induces strong acidification of A. castellanii cytoplasm (9 h.p.i.), even in the presence of bafilomycin A1. Scale bars, 10 µm. c, d The silencing of the canonical autophagy protein Atg8-2 does not prevent rRNA shutdown induced by Tupanvirus infection. Error bars, standard deviation. e Electron microscopy of A. castellanii infected by Tupanvirus (2 h.p.i.), highlighting the degradation of the nucleolus, nuclear disorganization and the formation of ribosome-containing vesicles near nuclear membranes. Scale bar, 500 nm. Red arrow: single-membrane vesicles containing ribosomes; orange arrow: double-membraned vesicles containing ribosomes; asterisk: ribosomes wrapping by the external nuclear membrane. Right: electron microscopy of uninfected amoebae and APMV infected cell (8 h.p.i.) showing a mild nucleolar disorganization in the presence of the virus. Scale bars: 1 µm. f Haemacolour staining showing the nuclear degradation of A. castellanii induced by Tupanvirus infection (9 h.p.i.) compared with infection by mimivirus (APMV) and uninfected cells. Tupanvirus-infected cells are purplish because of cytoplasm acidification. Scale bars, 5 µm. Arrows: nucleus, when present; VF: viral factory. g Haemacolour staining showing the nuclear degradation in Tetrahymena sp. induced by Tupanvirus infection (4 days post-inoculation) compared with mimivirus (APMV)-inoculated cells and uninfected cells. Tupanvirus-infected cells present an atypical shape and intense vacuolization, and some cells lack a nucleus (asterisk). Arrows: nucleus under degradation. Scale bars, 5 µm. The experiments were performed 3 times independently, with two replicates each
Fig. 8
rRNA shutdown induced by Tupanvirus and toxicity assays. a Tupanvirus and mimivirus titers (log10 values) 24 h.p.i. in Acanthamoeba castellanii at distinct MOIs. b Ribosomal 18S RNA relative measure by qPCR from A. castellanii infected by tupanvirus or mimivirus at an MOI of 10 or 100, 3 and 9 h post-infection. c Electrophoresis gel showing ribosomal 18S and 28S RNA from A. castellanii under the same conditions described in b. d Vesicle containing a large amount of A. castellanii ribosomes (R) 6 h after Tupanvirus infection. Scale bar, 1 µm. (e) Cytopathic effect of A. castellanii inoculated with Tupanvirus or mimivirus after UV or heat inactivation, MOI of 100, 8 h post-inoculation. Scale bar, 20 µm. f Counting of A. castellanii presenting cytopathic effect 8 h post-inoculation with tupanvirus inactivated by UV or heating under different MOIs. g Ribosomal 18S RNA relative measure by qPCR from A. castellanii infected by UV-inativated or heat-inactivated Tupanvirus, or APMV, at an MOI of 100, 3 and 9 h post-infection. h Dose–response of Tupanvirus and APMV in A. castellanii pre-treated with distinct doses of geneticin or cycloheximide. (i) Progressive vacuolization of tetrahymena after infection with Tupanvirus (days 1–4). Scale bars, 10 µm. j Tupanvirus tail content releasing in tetrahymena 1 h post-inoculation (TEM). Scale bar, 200 nm. k Vesicles containing large amounts of Tetrahymena ribosomes (R) after Tupanvirus infection. Scale bar, 3.5 µm. l Electrophoresis gel showing rRNA shutdown in tetrahymena inoculated with Tupanvirus at an MOI of 10 (days 1–4). m Rate of particles incorporation per tetrahymena cell (days 1–4). n Simulations showing the decrease in APMV and maintenance of Tupanvirus populations over the analyzed days after infection of a mix of Acanthamoeba castellanii and tetrahymena at an MOI of 10 (lines indicated by ‘b’ in both graphs of n). At days 4 and 8, fresh PYG medium and 105 A. castellanii were added to the systems (arrows). For the negative control of this experiment we pre-treated tetrahymena with 20 µg/ml of geneticin (lines indicated by ‘a’ in both graphs of n). In this case, both APMV and Tupanvirus were able to grow in the system. Statistical analyses in b and h: t-test based on control groups (b) or corresponding virus/drug concentrations (h). *:p < 0.05; **:p < 0.01. The experiments were performed 3 times independently, with two replicates each. Error bars (a, b, f, g, h, m and n), standard deviation
Similar articles
- Translating the language of giants: translation-related genes as a major contribution of giant viruses to the virosphere.
Rodrigues RAL, da Silva LCF, Abrahão JS. Rodrigues RAL, et al. Arch Virol. 2020 Jun;165(6):1267-1278. doi: 10.1007/s00705-020-04626-2. Epub 2020 Apr 24. Arch Virol. 2020. PMID: 32333117 Review. - "Tupanvirus", a new genus in the family Mimiviridae.
Rodrigues RAL, Mougari S, Colson P, La Scola B, Abrahão JS. Rodrigues RAL, et al. Arch Virol. 2019 Jan;164(1):325-331. doi: 10.1007/s00705-018-4067-4. Epub 2018 Oct 5. Arch Virol. 2019. PMID: 30291500 - The Complex Nature of Tupanviruses.
Rodrigues RAL, Arantes TS, Oliveira GP, Dos Santos Silva LK, Abrahão JS. Rodrigues RAL, et al. Adv Virus Res. 2019;103:135-166. doi: 10.1016/bs.aivir.2018.09.001. Epub 2018 Nov 10. Adv Virus Res. 2019. PMID: 30635075 Review. - Morphological and Taxonomic Properties of the Newly Isolated Cotonvirus japonicus, a New Lineage of the Subfamily Megavirinae.
Takahashi H, Fukaya S, Song C, Murata K, Takemura M. Takahashi H, et al. J Virol. 2021 Aug 25;95(18):e0091921. doi: 10.1128/JVI.00919-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34191583 Free PMC article. - Isolation of Yasminevirus, the First Member of Klosneuvirinae Isolated in Coculture with Vermamoeba vermiformis, Demonstrates an Extended Arsenal of Translational Apparatus Components.
Bajrai LH, Mougari S, Andreani J, Baptiste E, Delerce J, Raoult D, Azhar EI, La Scola B, Levasseur A. Bajrai LH, et al. J Virol. 2019 Dec 12;94(1):e01534-19. doi: 10.1128/JVI.01534-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597770 Free PMC article.
Cited by
- Origin of viruses: primordial replicators recruiting capsids from hosts.
Krupovic M, Dolja VV, Koonin EV. Krupovic M, et al. Nat Rev Microbiol. 2019 Jul;17(7):449-458. doi: 10.1038/s41579-019-0205-6. Nat Rev Microbiol. 2019. PMID: 31142823 Review. - A giant virus infecting the amoeboflagellate Naegleria.
Arthofer P, Panhölzl F, Delafont V, Hay A, Reipert S, Cyran N, Wienkoop S, Willemsen A, Sifaoui I, Arberas-Jiménez I, Schulz F, Lorenzo-Morales J, Horn M. Arthofer P, et al. Nat Commun. 2024 Apr 24;15(1):3307. doi: 10.1038/s41467-024-47308-2. Nat Commun. 2024. PMID: 38658525 Free PMC article. - The Role of Tape Measure Protein in Nucleocytoplasmic Large DNA Virus Capsid Assembly.
Xian Y, Avila R, Pant A, Yang Z, Xiao C. Xian Y, et al. Viral Immunol. 2021 Jan-Feb;34(1):41-48. doi: 10.1089/vim.2020.0038. Epub 2020 Oct 19. Viral Immunol. 2021. PMID: 33074779 Free PMC article. - A survey of RNA viruses in mosquitoes from Mozambique reveals novel genetic lineages of flaviviruses and phenuiviruses, as well as frequent flavivirus-like viral DNA forms in Mansonia.
Abílio AP, Silva M, Kampango A, Narciso I, Gudo ES, das Neves LCB, Sidat M, Fafetine JM, de Almeida APG, Parreira R. Abílio AP, et al. BMC Microbiol. 2020 Jul 28;20(1):225. doi: 10.1186/s12866-020-01905-5. BMC Microbiol. 2020. PMID: 32723369 Free PMC article. - Hidden diversity of soil giant viruses.
Schulz F, Alteio L, Goudeau D, Ryan EM, Yu FB, Malmstrom RR, Blanchard J, Woyke T. Schulz F, et al. Nat Commun. 2018 Nov 19;9(1):4881. doi: 10.1038/s41467-018-07335-2. Nat Commun. 2018. PMID: 30451857 Free PMC article.
References
- Korobeinikova AV, Garber MB, Gongadze GM. Ribosomal proteins: structure, function, and evolution. Biochemistry. 2012;77:562–574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous