The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects - PubMed (original) (raw)
Review
The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects
Farhad Dehkhoda et al. Front Endocrinol (Lausanne). 2018.
Abstract
The growth hormone receptor (GHR), although most well known for regulating growth, has many other important biological functions including regulating metabolism and controlling physiological processes related to the hepatobiliary, cardiovascular, renal, gastrointestinal, and reproductive systems. In addition, growth hormone signaling is an important regulator of aging and plays a significant role in cancer development. Growth hormone activates the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway, and recent studies have provided a new understanding of the mechanism of JAK2 activation by growth hormone binding to its receptor. JAK2 activation is required for growth hormone-mediated activation of STAT1, STAT3, and STAT5, and the negative regulation of JAK-STAT signaling comprises an important step in the control of this signaling pathway. The GHR also activates the Src family kinase signaling pathway independent of JAK2. This review covers the molecular mechanisms of GHR activation and signal transduction as well as the physiological consequences of growth hormone signaling.
Keywords: Janus kinase 2; Src family kinase; growth hormone; growth hormone receptor; insulin-like growth factor 1; suppressor of cytokine signaling.
Figures
Figure 1
The growth hormone receptor domain organization.
Figure 2
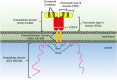
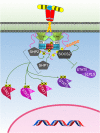
Activation of the growth hormone receptor (GHR) by growth hormone. In the inactive homodimeric GHR, (A) the Janus kinase (JAK) 2 kinase domain (KD) is inhibited in trans by interaction with the pseudokinase domain from the JAK2 bound to the opposing receptor within the homodimer. GH binding to the GHR extracellular domain (B) results in conformational changes that cause the transmembrane domains to transition from a parallel interaction to a left-handed crossover interaction. These structural changes cause a separation of the intracellular domains to the Box1 and Box2 motifs and the associated JAK2 molecules. The movement of the associated JAK2s dissociates the inhibitory interaction of the pseudokinase from the KD and brings the two JAK2 KDs in close proximity resulting in trans phosphorylation and activation.
Figure 3
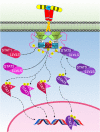
The Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway initiated by the activated growth hormone receptor. Activated JAK2 phosphorylates tyrosines on the intracellular domain of the receptor. Inactive-STAT5 dimers bind these phosphorylated tyrosine residues on the receptor, and the STAT5 is subsequently phosphorylated by JAK2 forming different active-STAT5 dimers that are translocated to the nucleus, bind DNA, and act as transcription factors. STAT1 and STAT3 are phosphorylated and activated by JAK2. Active STAT1 and STAT3 form homodimers or heterodimers, are translocated to the nucleus, bind DNA, and act as transcription factors.
Figure 4
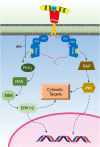
The Src family kinase (SFK) signaling initiated by the activated growth hormone receptor (GHR). SFKs are activated by GH binding to GHR. This signaling pathway activates ERK1/2 that regulates cytosolic targets and gene transcription.
Figure 5
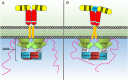
The major negative regulation of growth hormone receptor (GHR) signaling. GH-activated GHR results in suppressor of cytokine signaling (SOCS) 2 binding to a phosphorylated tyrosine on the GHR intracellular domain. This blocks signal transducer and activator of transcription (STAT) 5 binding and induces ubiquitination of the GHR leading to targeted degradation of the receptor. SHP1 and SHP2 act to dephosphorylate and inactivate Janus kinase 2 and STATs, while SHP2 is known to also dephosphorylate the receptor.
Figure 6
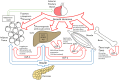
Organ-specific roles of the GH/insulin-like growth factor-1 (IGF-1) axis.
Similar articles
- Downregulation of the growth hormone-induced Janus kinase 2/signal transducer and activator of transcription 5 signaling pathway requires an intact actin cytoskeleton.
Rico-Bautista E, Negrín-Martínez C, Novoa-Mogollón J, Fernández-Perez L, Flores-Morales A. Rico-Bautista E, et al. Exp Cell Res. 2004 Mar 10;294(1):269-80. doi: 10.1016/j.yexcr.2003.11.017. Exp Cell Res. 2004. PMID: 14980520 - The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH.
Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. Smit LS, et al. Mol Endocrinol. 1996 May;10(5):519-33. doi: 10.1210/mend.10.5.8732683. Mol Endocrinol. 1996. PMID: 8732683 - In vivo targeting of the growth hormone receptor (GHR) Box1 sequence demonstrates that the GHR does not signal exclusively through JAK2.
Barclay JL, Kerr LM, Arthur L, Rowland JE, Nelson CN, Ishikawa M, d'Aniello EM, White M, Noakes PG, Waters MJ. Barclay JL, et al. Mol Endocrinol. 2010 Jan;24(1):204-17. doi: 10.1210/me.2009-0233. Epub 2009 Nov 2. Mol Endocrinol. 2010. PMID: 19884384 Free PMC article. - Role of the JAK-STAT Pathway in Bovine Mastitis and Milk Production.
Khan MZ, Khan A, Xiao J, Ma Y, Ma J, Gao J, Cao Z. Khan MZ, et al. Animals (Basel). 2020 Nov 13;10(11):2107. doi: 10.3390/ani10112107. Animals (Basel). 2020. PMID: 33202860 Free PMC article. Review.
Cited by
- Monomerization of Homodimeric Trefoil Factor 3 (TFF3) by an Aminonitrile Compound Inhibits TFF3-Dependent Cancer Cell Survival.
Pandey V, Zhang X, Poh HM, Wang B, Dukanya D, Ma L, Yin Z, Bender A, Periyasamy G, Zhu T, Rangappa KS, Basappa B, Lobie PE. Pandey V, et al. ACS Pharmacol Transl Sci. 2022 Aug 17;5(9):761-773. doi: 10.1021/acsptsci.2c00044. eCollection 2022 Sep 9. ACS Pharmacol Transl Sci. 2022. PMID: 36110371 Free PMC article. - Association of DNA-Methylation Profiles With Immune Responses Elicited in Breast Cancer Patients Immunized With a Carbohydrate-Mimicking Peptide: A Pilot Study.
Hernandez Puente CV, Hsu PC, Rogers LJ, Jousheghany F, Siegel E, Kadlubar SA, Beck JT, Makhoul I, Hutchins LF, Kieber-Emmons T, Monzavi-Karbassi B. Hernandez Puente CV, et al. Front Oncol. 2020 Jun 5;10:879. doi: 10.3389/fonc.2020.00879. eCollection 2020. Front Oncol. 2020. PMID: 32582547 Free PMC article. - Evaluating the local expression pattern of IGF-1R in tumor tissues and the circulating levels of IGF-1, IGFBP-1, and IGFBP-3 in the blood of patients with different primary bone tumors.
Vaezi MA, Eghtedari AR, Safizadeh B, Babaheidarian P, Salimi V, Adjaminezhad-Fard F, Yarahmadi S, Mirzaei A, Rahbar M, Tavakoli-Yaraki M. Vaezi MA, et al. Front Oncol. 2023 Jan 13;12:1096438. doi: 10.3389/fonc.2022.1096438. eCollection 2022. Front Oncol. 2023. PMID: 36713521 Free PMC article. - The growth hormone receptor interacts with transcriptional regulator HMGN1 upon GH-induced nuclear translocation.
Jain L, Vickers MH, Jacob B, Middleditch MJ, Chudakova DA, Ganley ARD, O'Sullivan JM, Perry JK. Jain L, et al. J Cell Commun Signal. 2023 Sep;17(3):925-937. doi: 10.1007/s12079-023-00741-2. Epub 2023 Apr 12. J Cell Commun Signal. 2023. PMID: 37043098 Free PMC article. - Classical and novel GH receptor signaling pathways.
Frank SJ. Frank SJ. Mol Cell Endocrinol. 2020 Dec 1;518:110999. doi: 10.1016/j.mce.2020.110999. Epub 2020 Aug 22. Mol Cell Endocrinol. 2020. PMID: 32835785 Free PMC article. Review.
References
- Brooks AJ, Dehkhoda F, Kragelund BB. Cytokine receptors. In: Belfiore A, LeRoith D, editors. Principles of Endocrinology and Hormone Action. Springer International Publishing; (2016). p. 1–29.
- Baumgartner JW, Wells CA, Chen CM, Waters MJ. The role of the WSXWS equivalent motif in growth hormone receptor function. J Biol Chem (1994) 269(46):29094–101. - PubMed
- Hamming OJ, Kang L, Svensson A, Karlsen JL, Rahbek-Nielsen H, Paludan SR, et al. Crystal structure of interleukin-21 receptor (IL-21R) bound to IL-21 reveals that sugar chain interacting with WSXWS motif is integral part of IL-21R. J Biol Chem (2012) 287(12):9454–60.10.1074/jbc.M111.311084 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous