SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model - PubMed (original) (raw)
SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model
Laura Bonfili et al. Mol Neurobiol. 2018 Oct.
Abstract
The gut-brain axis is a bidirectional communication network functionally linking the gut and the central nervous system (CNS). Based on this, the rational manipulation of intestinal microbiota represents a novel attractive therapeutic strategy for the treatment of CNS-associated disorders. In this study, we explored the properties of a probiotic formulation (namely SLAB51) in counteracting brain oxidative damages associated with Alzheimer's disease (AD). Specifically, transgenic AD mice (3xTg-AD) were treated with SLAB51 and the effects on protein oxidation, neuronal antioxidant defence and repair systems were monitored, with the particular focus on the role of SIRT1-related pathways. We demonstrated that SLAB51 markedly reduced oxidative stress in AD mice brain by activating SIRT1-dependent mechanisms, thus representing a promising therapeutic adjuvant in AD treatment.
Keywords: Alzheimer’s disease; Oxidation; Probiotics; SIRT1.
Figures
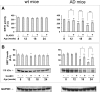
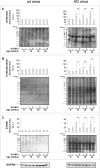
Fig. 1
Effect of SLAB51 on SIRT1 activity and expression. SIRT1 activity (a) and expression levels (b) measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice. The enzyme activity is expressed as fluorescent units (F.U.). The densitometric analyses obtained from five separate blots and representative immunoblots are shown. Equal protein loading was verified by using an anti-GAPDH antibody. The detection was performed with an ECL Western blotting analysis system. Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively
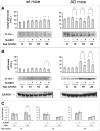
Fig. 2
Effect of SLAB51 on p53. Acetylated p53 (a) and p53 (b) levels measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice. The densitometric analyses obtained from five separate blots and representative immunoblots are shown. Equal protein loading was verified by using an anti-GAPDH antibody. The detection was performed with an ECL Western blotting analysis system. Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively. (c) Pairwise comparison of the effects of SLAB51 in Ac-p53/p53 ratios in wt and AD ageing mice. Statistical significance compared to age-matched mice is indicated with “§” mark (§p < 0.05; §§p < 0.01; §§§p < 0.001)
Fig. 3
Effect of SLAB51 on RARβ. Acetylated RARβ (a) and RARβ (b) levels measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice. The densitometric analyses obtained from five separate blots and representative immunoblots are shown. Equal protein loading was verified by using an anti-GAPDH antibody. The detection was performed with an ECL Western blotting analysis system. Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively. c: Pairwise comparison of the effects of SLAB51 in Ac-RARβ/RARβ in wt and AD ageing mice. Statistical significance compared to age-matched mice is indicated with “§” mark (§p < 0.05; §§p < 0.01; §§§p < 0.001)
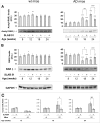
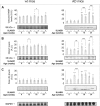
Fig. 4
Effect of SLAB51 on the activity of antioxidant enzymes. GST (a), GPx (b), SOD (c) and CAT (d) activities measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice (see “Materials and Methods” section for further details). Results are expressed as fluorescence units (F.U.). Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively
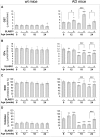
Fig. 5
Effect of SLAB51 on protein and lipid oxidation. Protein carbonyls (a), 3-NT (b) and 4-HNE adduct (c) levels measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice. The densitometric analyses obtained from five separate blots and representative immunoblots are shown. Equal protein loading for 3-NT (b) and 4-HNE adducts (c) was verified by using an anti-GAPDH antibody. Ponceau staining has been used to check loading in oxyblot, as reported in the “Materials and Methods” section (a, staining not shown). The detection was performed with an ECL Western blotting analysis system. Molecular weight standards (6–205 kDa) were used for molar mass calibration (myosin 205 kDa, β-galactosidase 116 kDa, phosphorylase b 97 kDa, fructose-6-phosphate kinase 80 kDa, albumin 66 kDa, glutamic dehydrogenase 55 kDa, ovalbumin 45 kDa, carbonic anhydrase 30 kDa, trypsin inhibitor 21 kDa, lysozyme 14 kDa, aprotinin 6.5 kDa). Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively
Fig. 6
Effect of SLAB51 on DNA oxidation and repair mechanisms. Cleaved PARP (a), OGG1 (b) and 8-oxodG (c) levels measured in brain homogenates of SLAB51-treated and SLAB51-untreated wt (left) and AD (right) mice. The densitometric analyses obtained from five separate blots and representative immunoblots are shown. Equal protein loading was verified by using an anti-GAPDH antibody. The detection was performed with an ECL Western blotting analysis system. Statistical significance compared to untreated 8-week-old mice and age-matched mice is indicated with asterisks (*p < 0.05; **p < 0.01; ***p < 0.001) and hashtags (#p < 0.05; ##p < 0.01; ###p < 0.001), respectively
Similar articles
- Gut microbiota modulation in Alzheimer's disease: Focus on lipid metabolism.
Bonfili L, Cuccioloni M, Gong C, Cecarini V, Spina M, Zheng Y, Angeletti M, Eleuteri AM. Bonfili L, et al. Clin Nutr. 2022 Mar;41(3):698-708. doi: 10.1016/j.clnu.2022.01.025. Epub 2022 Feb 2. Clin Nutr. 2022. PMID: 35158177 - Neuroprotective Mechanisms of Resveratrol in Alzheimer's Disease: Role of SIRT1.
Gomes BAQ, Silva JPB, Romeiro CFR, Dos Santos SM, Rodrigues CA, Gonçalves PR, Sakai JT, Mendes PFS, Varela ELP, Monteiro MC. Gomes BAQ, et al. Oxid Med Cell Longev. 2018 Oct 30;2018:8152373. doi: 10.1155/2018/8152373. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30510627 Free PMC article. Review. - Strategic Modification of Gut Microbiota through Oral Bacteriotherapy Influences Hypoxia Inducible Factor-1α: Therapeutic Implication in Alzheimer's Disease.
Bonfili L, Gong C, Lombardi F, Cifone MG, Eleuteri AM. Bonfili L, et al. Int J Mol Sci. 2021 Dec 29;23(1):357. doi: 10.3390/ijms23010357. Int J Mol Sci. 2021. PMID: 35008786 Free PMC article. - Neophobia, NQO1 and SIRT1 as premorbid and prodromal indicators of AD in 3xTg-AD mice.
Torres-Lista V, Parrado-Fernández C, Alvarez-Montón I, Frontiñán-Rubio J, Durán-Prado M, Peinado JR, Johansson B, Alcaín FJ, Giménez-Llort L. Torres-Lista V, et al. Behav Brain Res. 2014 Sep 1;271:140-6. doi: 10.1016/j.bbr.2014.04.055. Epub 2014 May 13. Behav Brain Res. 2014. PMID: 24837743 - Nutritional antioxidants and the heme oxygenase pathway of stress tolerance: novel targets for neuroprotection in Alzheimer's disease.
Calabrese V, Butterfield DA, Stella AM. Calabrese V, et al. Ital J Biochem. 2003 Dec;52(4):177-81. Ital J Biochem. 2003. PMID: 15141484 Review.
Cited by
- Protective effects of a lactobacilli mixture against Alzheimer's disease-like pathology triggered by Porphyromonas gingivalis.
Kazemi N, Khorasgani MR, Noorbakhshnia M, Razavi SM, Narimani T, Naghsh N. Kazemi N, et al. Sci Rep. 2024 Nov 8;14(1):27283. doi: 10.1038/s41598-024-77853-1. Sci Rep. 2024. PMID: 39516514 Free PMC article. - The Microbiota-Gut-Brain Axis and Neurological Disorders: A Comprehensive Review.
Nakhal MM, Yassin LK, Alyaqoubi R, Saeed S, Alderei A, Alhammadi A, Alshehhi M, Almehairbi A, Al Houqani S, BaniYas S, Qanadilo H, Ali BR, Shehab S, Statsenko Y, Meribout S, Sadek B, Akour A, Hamad MIK. Nakhal MM, et al. Life (Basel). 2024 Sep 26;14(10):1234. doi: 10.3390/life14101234. Life (Basel). 2024. PMID: 39459534 Free PMC article. Review. - Probiotics as Potential Tool to Mitigate Nucleotide Metabolism Alterations Induced by DiNP Dietary Exposure in Danio rerio.
Giommi C, Maradonna F, Ladisa C, Habibi HR, Carnevali O. Giommi C, et al. Int J Mol Sci. 2024 Oct 17;25(20):11151. doi: 10.3390/ijms252011151. Int J Mol Sci. 2024. PMID: 39456934 Free PMC article. - Gut microbiota metabolites: potential therapeutic targets for Alzheimer's disease?
Zhang S, Lu J, Jin Z, Xu H, Zhang D, Chen J, Wang J. Zhang S, et al. Front Pharmacol. 2024 Sep 17;15:1459655. doi: 10.3389/fphar.2024.1459655. eCollection 2024. Front Pharmacol. 2024. PMID: 39355779 Free PMC article. Review. - New insights in lipid metabolism: potential therapeutic targets for the treatment of Alzheimer's disease.
Cao Y, Zhao LW, Chen ZX, Li SH. Cao Y, et al. Front Neurosci. 2024 Sep 11;18:1430465. doi: 10.3389/fnins.2024.1430465. eCollection 2024. Front Neurosci. 2024. PMID: 39323915 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical