Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson's disease - PubMed (original) (raw)
Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson's disease
Etheresia Pretorius et al. PLoS One. 2018.
Abstract
The thrombin-induced polymerisation of fibrinogen to form fibrin is well established as a late stage of blood clotting. It is known that Parkinson's Disease (PD) is accompanied by dysregulation in blood clotting, but it is less widely known as a coagulopathy. In recent work, we showed that the presence of tiny amounts of bacterial lipopolysaccharide (LPS) in healthy individuals could cause clots to adopt an amyloid form, and this could be observed via scanning electron microscopy (SEM) or via the fluorescence of thioflavin-T. This could be prevented by the prior addition of lipopolysaccharide-binding protein (LBP). We had also observed by SEM this unusual clotting in the blood of patients with Parkinson's Disease. We hypothesised, and here show, that this too can be prevented by LBP in the context of PD. This adds further evidence implicating inflammatory microbial cell wall products as an accompaniment to the disease, and may be part of its aetiology. This may lead to novel treatment strategies in PD designed to target microbes and their products.
Conflict of interest statement
Competing Interests: The authors declare the following patent application: Method for treating Parkinson’s Disease (ZA2017/05547).
Figures
Fig 1
A and B) Clot structure from two healthy individuals, imaged in an SEM and showing a ‘normal’ ‘spaghetti’ or ‘noodle’ type of structure. All clots were created by adding thrombin to PPP. C) SEM micrographs of PPP from a Parkinson’s disease individuals with added thrombin, showing a ‘dense matted deposit’; D) PPP from same individual, but exposed to 2ng.L-1 LPS-binding protein followed by addition of thrombin; now showing a structure similar to that of healthy controls.
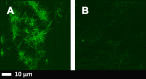
Fig 2
A): Airyscan micrographs of PPP with added thrombin to form extensive fibrin fibres from an individual with Parkinson’s disease (PD); B) PPP from the same individual, but exposed to 2ng.L-1 LPS-binding protein followed by addition of thrombin. Thioflavin T (ThT) (5 μM) was added before thrombin. Micrographs were taken with a Zeiss LSM 510 META confocal microscope with a Plan-Apochromat 63x/1.4 Oil DIC objective. LBP dramatically reduced the fluorescence seen in samples from patients with PD. Gain settings were kept the same during all data capturing and not changed for statistical analysis, but brightness and contrast was slightly adjusted for fig preparation.
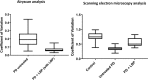
Fig 3. Boxplots of the distribution of the coefficients of variation in the pixel intensities of the SEM and Airyscan clot images.
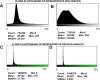
Fig 4
A and B) Representative histograms of the 8-bit intensity for a representative SEM clot from PPP of an individual with Parkinson’s disease and after addition of LBP, respectively. C and D) Representative histograms of the 8-bit intensity for a typical Airyscan clot from PPP of an individual with Parkinson’s disease and after addition of LBP, respectively.
Similar articles
- Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular co-morbidities.
Pretorius E, Mbotwe S, Kell DB. Pretorius E, et al. Sci Rep. 2017 Aug 29;7(1):9680. doi: 10.1038/s41598-017-09860-4. Sci Rep. 2017. PMID: 28851981 Free PMC article. - Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson's Disease, Alzheimer's Disease and Type 2 Diabetes Mellitus.
de Waal GM, Engelbrecht L, Davis T, de Villiers WJS, Kell DB, Pretorius E. de Waal GM, et al. Sci Rep. 2018 Nov 14;8(1):16798. doi: 10.1038/s41598-018-35009-y. Sci Rep. 2018. PMID: 30429533 Free PMC article. - Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide.
Pretorius E, Mbotwe S, Bester J, Robinson CJ, Kell DB. Pretorius E, et al. J R Soc Interface. 2016 Sep;13(122):20160539. doi: 10.1098/rsif.2016.0539. J R Soc Interface. 2016. PMID: 27605168 Free PMC article. - The role of lipopolysaccharide-binding protein in modulating the innate immune response.
Zweigner J, Schumann RR, Weber JR. Zweigner J, et al. Microbes Infect. 2006 Mar;8(3):946-52. doi: 10.1016/j.micinf.2005.10.006. Epub 2006 Jan 13. Microbes Infect. 2006. PMID: 16483818 Review. - A novel acute-phase marker: lipopolysaccharide binding protein (LBP).
Schumann RR, Zweigner J. Schumann RR, et al. Clin Chem Lab Med. 1999 Mar;37(3):271-4. doi: 10.1515/CCLM.1999.047. Clin Chem Lab Med. 1999. PMID: 10353471 Review.
Cited by
- Proteomic Study of a Parkinson's Disease Model of Undifferentiated SH-SY5Y Cells Induced by a Proteasome Inhibitor.
Jiang H, Yu Y, Liu S, Zhu M, Dong X, Wu J, Zhang Z, Zhang M, Zhang Y. Jiang H, et al. Int J Med Sci. 2019 Jan 1;16(1):84-92. doi: 10.7150/ijms.28595. eCollection 2019. Int J Med Sci. 2019. PMID: 30662332 Free PMC article. - Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis.
Mikołajczyk A, Złotkowska D. Mikołajczyk A, et al. Int J Mol Sci. 2018 Oct 22;19(10):3274. doi: 10.3390/ijms19103274. Int J Mol Sci. 2018. PMID: 30360353 Free PMC article. - A Perspective on How Fibrinaloid Microclots and Platelet Pathology May be Applied in Clinical Investigations.
Pretorius E, Kell DB. Pretorius E, et al. Semin Thromb Hemost. 2024 Jun;50(4):537-551. doi: 10.1055/s-0043-1774796. Epub 2023 Sep 25. Semin Thromb Hemost. 2024. PMID: 37748515 Free PMC article. Review. - Possible Role of Fibrinaloid Microclots in Postural Orthostatic Tachycardia Syndrome (POTS): Focus on Long COVID.
Kell DB, Khan MA, Kane B, Lip GYH, Pretorius E. Kell DB, et al. J Pers Med. 2024 Jan 31;14(2):170. doi: 10.3390/jpm14020170. J Pers Med. 2024. PMID: 38392604 Free PMC article. - Proteomic Evidence for Amyloidogenic Cross-Seeding in Fibrinaloid Microclots.
Kell DB, Pretorius E. Kell DB, et al. Int J Mol Sci. 2024 Oct 8;25(19):10809. doi: 10.3390/ijms251910809. Int J Mol Sci. 2024. PMID: 39409138 Free PMC article.
References
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901 . - DOI - PubMed
- Herczenik E, Gebbink MFBG. Molecular and cellular aspects of protein misfolding and disease. FASEB J. 2008;22(7):2115–33. doi: 10.1096/fj.07-099671 . - DOI - PubMed
- Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–203. doi: 10.1016/j.cell.2012.02.022 ; PubMed Central PMCID: PMCPMC3353745. - DOI - PMC - PubMed
- Knowles TPJ, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15(6):384–96. doi: 10.1038/nrm3810 PubMed PMID: WOS:000337245500010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the National Research Foundation grant 91548: Competitive Program and South African Medical Research Council to EP, and the Biotechnology and Biological Sciences Research Council Grant BB/L025752/1 to DBK. Some reagent funds were supplied as a philanthropic gift by an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous