Sex differences in gut microbiota in patients with major depressive disorder - PubMed (original) (raw)
Sex differences in gut microbiota in patients with major depressive disorder
Jian-Jun Chen et al. Neuropsychiatr Dis Treat. 2018.
Abstract
Objective: Our previous studies found that disturbances in gut microbiota might have a causative role in the onset of major depressive disorder (MDD). The aim of this study was to investigate whether there were sex differences in gut microbiota in patients with MDD.
Patients and methods: First-episode drug-naïve MDD patients and healthy controls were included. 16S rRNA gene sequences extracted from the fecal samples of the included subjects were analyzed. Principal-coordinate analysis and partial least squares-discriminant analysis were used to assess whether there were sex-specific gut microbiota. A random forest algorithm was used to identify the differential operational taxonomic units. Linear discriminant-analysis effect size was further used to identify the dominant sex-specific phylotypes responsible for the differences between MDD patients and healthy controls.
Results: In total, 57 and 74 differential operational taxonomic units responsible for separating female and male MDD patients from their healthy counterparts were identified. Compared with their healthy counterparts, increased Actinobacteria and decreased Bacteroidetes levels were found in female and male MDD patients, respectively. The most differentially abundant bacterial taxa in female and male MDD patients belonged to phyla Actinobacteria and Bacteroidia, respectively. Meanwhile, female and male MDD patients had different dominant phylotypes.
Conclusion: These results demonstrated that there were sex differences in gut microbiota in patients with MDD. The suitability of Actinobacteria and Bacteroidia as the sex-specific biomarkers for diagnosing MDD should be further explored.
Keywords: MDD; biomarker; gut microbiota; major depressive disorder.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Figure 1
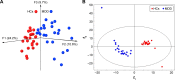
Obvious differences in gut microbial composition between female MDD patients and HCs. Notes: (A) Three-dimensional principal-coordinate analysis; (B) partial least squares-discriminant analysis. Abbreviations: MDD, major depressive disorder; HCs, healthy controls.
Figure 2
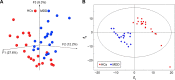
Obvious differences in gut microbial composition between male MDD patients and HCs. Notes: (A) Three-dimensional principal-coordinate analysis; (B) partial least squares-discriminant analysis. Abbreviations: MDD, major depressive disorder; HCs, healthy controls.
Figure 3
Heat map of differential operational taxonomic unit abundance between female MDD patients and HCs. Notes: Assignment of each operational taxonomic unit provided at right. Green and red indicate increase and decrease, respectively. Abbreviations: MDD, major depressive disorder; HCs, healthy controls.
Figure 4
Heat map of differential operational taxonomic unit abundance between male MDD patients and HCs. Notes: Assignment of each operational taxonomic unit provided at right. Green and red indicate increase and decrease, respectively. Abbreviations: MDD, major depressive disorder; HCs, healthy controls.
Figure 5
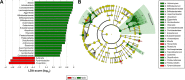
Taxonomic differences in gut microbiota in female subjects. Notes: (A) Bacterial clades (25) with statistically significant and biologically consistent differences in female MDD patients and HCs (LDA score >2); (B) MDD-enriched taxa indicated by positive LDA scores (green), and HC-enriched taxa indicated by negative scores (red). Abbreviations: MDD, major depressive disorder; HCs, healthy controls; LDA, linear discriminant analysis.
Figure 6
Taxonomic differences in gut microbiota in male subjects. Notes: (A) Twelve bacterial clades with statistically significant and biologically consistent differences in male MDD patients and HCs (LDA score >2); (B) MDD-enriched taxa indicated by positive LDA scores (green), and HC-enriched taxa indicated by negative scores (red). Abbreviations: MDD, major depressive disorder; HCs, healthy controls; LDA, linear discriminant analysis.
Figure 7
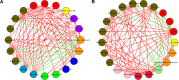
Associations among demographic data (age, BMI, and HDRS) and gut microbiota. Notes: (A) Female and (B) male MDD patients. Red lines indicate positive relationships, green lines negative relationship, and circles: red, demographic data; green, Asaccharobacter; blue, Clostridium XIVa; orange, Erysipelotrichaceae incertae sedis; dark orchid, Faecalibacterium; yellow, Lachnospiraceae incertae sedis; deep sky blue, Streptococcus; crimson, Collinsella; light green, Dorea; pink, Veillonella; olive, others. Abbreviations: BMI, body-mass index; HDRS, Hamilton Depression Rating Scale; MDD, major depressive disorder.
Similar articles
- Age-specific differential changes on gut microbiota composition in patients with major depressive disorder.
Chen JJ, He S, Fang L, Wang B, Bai SJ, Xie J, Zhou CJ, Wang W, Xie P. Chen JJ, et al. Aging (Albany NY). 2020 Feb 10;12(3):2764-2776. doi: 10.18632/aging.102775. Epub 2020 Feb 10. Aging (Albany NY). 2020. PMID: 32040443 Free PMC article. - Characteristics of gut microbiota and its correlation with hs-CRP and somatic symptoms in first-episode treatment-naive major depressive disorder.
Liu P, Jing L, Guo F, Xu Y, Cheng J, Liu S, Liu L, Liu Z, Zhang K, Sun N. Liu P, et al. J Affect Disord. 2024 Jul 1;356:664-671. doi: 10.1016/j.jad.2024.04.011. Epub 2024 Apr 12. J Affect Disord. 2024. PMID: 38615845 - Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients.
Lai WT, Deng WF, Xu SX, Zhao J, Xu D, Liu YH, Guo YY, Wang MB, He FS, Ye SW, Yang QF, Liu TB, Zhang YL, Wang S, Li MZ, Yang YJ, Xie XH, Rong H. Lai WT, et al. Psychol Med. 2021 Jan;51(1):90-101. doi: 10.1017/S0033291719003027. Epub 2019 Nov 5. Psychol Med. 2021. PMID: 31685046 - Gut microbiota variations in patients diagnosed with major depressive disorder-A systematic review.
Knudsen JK, Bundgaard-Nielsen C, Hjerrild S, Nielsen RE, Leutscher P, Sørensen S. Knudsen JK, et al. Brain Behav. 2021 Jul;11(7):e02177. doi: 10.1002/brb3.2177. Epub 2021 May 28. Brain Behav. 2021. PMID: 34047485 Free PMC article. Review. - Major Depressive Disorder and gut microbiota - Association not causation. A scoping review.
Łoniewski I, Misera A, Skonieczna-Żydecka K, Kaczmarczyk M, Kaźmierczak-Siedlecka K, Misiak B, Marlicz W, Samochowiec J. Łoniewski I, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 2;106:110111. doi: 10.1016/j.pnpbp.2020.110111. Epub 2020 Sep 23. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32976952 Review.
Cited by
- Narrative Review: Pathogenesis of the Inflammatory Response and Intestinal Flora in Depression.
Zeng JW, Zhao JL, Han ZJ, Duan YJ, Lin L. Zeng JW, et al. Neuropsychiatr Dis Treat. 2023 Nov 16;19:2469-2483. doi: 10.2147/NDT.S430444. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 38029049 Free PMC article. Review. - Sex difference in prebiotics on gut and blood-brain barrier dysfunction underlying stress-induced anxiety and depression.
Jiang J, Fu Y, Tang A, Gao X, Zhang D, Shen Y, Mou T, Hu S, Gao J, Lai J. Jiang J, et al. CNS Neurosci Ther. 2023 Jun;29 Suppl 1(Suppl 1):115-128. doi: 10.1111/cns.14091. Epub 2023 Jan 17. CNS Neurosci Ther. 2023. PMID: 36650644 Free PMC article. - The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies.
Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, Müller DJ. Alli SR, et al. Int J Mol Sci. 2022 Apr 19;23(9):4494. doi: 10.3390/ijms23094494. Int J Mol Sci. 2022. PMID: 35562885 Free PMC article. Review. - From-Toilet-to-Freezer: A Review on Requirements for an Automatic Protocol to Collect and Store Human Fecal Samples for Research Purposes.
Widjaja F, Rietjens IMCM. Widjaja F, et al. Biomedicines. 2023 Sep 28;11(10):2658. doi: 10.3390/biomedicines11102658. Biomedicines. 2023. PMID: 37893032 Free PMC article. Review. - Sex Differences in the Gut-Brain Axis: Implications for Mental Health.
Holingue C, Budavari AC, Rodriguez KM, Zisman CR, Windheim G, Fallin MD. Holingue C, et al. Curr Psychiatry Rep. 2020 Nov 20;22(12):83. doi: 10.1007/s11920-020-01202-y. Curr Psychiatry Rep. 2020. PMID: 33216233 Free PMC article. Review.
References
- Chen JJ, Zhou CJ, Zheng P, et al. Differential urinary metabolites related with the severity of major depressive disorder. Behav Brain Res. 2017;332:280–287. - PubMed
- Chen JJ, Zhao LB, Liu YY, Fan SH, Xie P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: a systematic review and multiple-treatments meta-analysis. Behav Brain Res. 2017;320:30–36. - PubMed
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. - PubMed
- Chen JJ, Zhou CJ, Liu Z, et al. Divergent urinary metabolic phenotypes between major depressive disorder and bipolar disorder identified by a combined GC-MS and NMR spectroscopic metabonomic approach. J Proteome Res. 2015;14(8):3382–3389. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources