Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice - PubMed (original) (raw)
doi: 10.1016/j.jhep.2018.02.024. Epub 2018 Mar 8.
Tian Lan 2, Liyan Zeng 3, Haihua Luo 1, Xiaoyu Yang 1, Na Li 1, Xiaojiao Chen 4, Zhanguo Liu 5, Rui Li 2, Sanda Win 6, Shuwen Liu 7, Hongwei Zhou 4, Bernd Schnabl 8, Yong Jiang 9, Neil Kaplowitz 6, Peng Chen 10
Affiliations
- PMID: 29524531
- PMCID: PMC6365016
- DOI: 10.1016/j.jhep.2018.02.024
Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice
Shenhai Gong et al. J Hepatol. 2018 Jul.
Abstract
Background & aims: Acetaminophen (APAP) induced hepatotoxicity is a leading cause of acute liver failure worldwide. It is well established that the liver damage induced by acetaminophen exhibits diurnal variation. However, the detailed mechanism for the hepatotoxic variation is not clear. Herein, we aimed to determine the relative contributions of gut microbiota in modulating the diurnal variation of hepatotoxicity induced by APAP.
Methods: Male Balb/C mice were treated with or without antibiotics and a single dose of orally administered APAP (300 mg/kg) at ZT0 (when the light is on-start of resting period) and ZT12 (when the light is off-start of active period).
Results: In agreement with previous findings, hepatic injury was markedly enhanced at ZT12 compared with ZT0. Interestingly, upon antibiotic treatment, ZT12 displayed a protective effect against APAP hepatotoxicity similar to ZT0. Moreover, mice that received the cecal content from ZT12 showed more severe liver damage than mice that received the cecal content from ZT0. 16S sequencing data revealed significant differences in the cecal content between ZT0 and ZT12 in the compositional level. Furthermore, metabolomic analysis showed that the gut microbial metabolites were also different between ZT0 and ZT12. Specifically, the level of 1-phenyl-1,2-propanedione (PPD) was significantly higher at ZT12 than ZT0. Treatment with PPD alone did not cause obvious liver damage. However, PPD synergistically enhanced APAP-induced hepatic injury in vivo and in vitro. Finally, we found Saccharomyces cerevisiae, which could reduce intestinal PPD levels, was able to markedly alleviate APAP-induced liver damage at ZT12.
Conclusions: The gut microbial metabolite PPD was responsible, at least in part, for the diurnal variation of hepatotoxicity induced by APAP by decreasing glutathione levels.
Lay summary: Acetaminophen (APAP) induced acute liver failure because of over dose is a leading public health problem. APAP-induced liver injury exhibits diurnal variation, specifically APAP causes more severe liver damage when taken at night compared with in the morning. Herein, we showed that gut microbial metabolite, 1-phenyl-1,2-propanedione is involved in the rhythmic hepatotoxicity induced by APAP, by depleting hepatic glutathione (an important antioxidant) levels. Our data suggest gut microbiota may be a potential target for reducing APAP-induced acute liver injury.
Keywords: Acetaminophen; Acute liver injury; Gut microbiota; Metabolomics.
Copyright © 2018 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: All authors have no potential conflicts of interest to declare.
Figures
Figure 1:
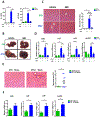
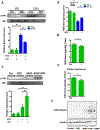
The diurnal variation of APAP induced hepatotoxicity was dependent on gut microbiota. Balb/C mice were treated with APAP for 24 hours with or without antibiotics pretreatment. (A) Plasma ALT, AST levels (n=6–12). (B) Representative gross liver appearance. (C) Hepatic HE staining and quantification of necrotic area (n=6–12). (D) mRNA levels of key cytokines and chemokines in the liver (n=6–12). (E) Mice received antibiotics intragastrically once daily for 5 days followed by receiving the cecal content from the donor mice (ZT0 or ZT12 group) once daily for 3 days. Mice were then gavaged with 300mg/kg APAP at ZT0 and were sacrificed 24 hours after APAP treatment. HE staining (n=6–12). (F) mRNA levels of key cytokines and chemokines in the liver (n=6–12). ZT0 = 8am. ZT12 = 8pm. The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05. Scale bar: 100 μm. N.S.: non-siginificance.
Figure 2:
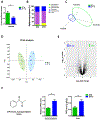
Diurnal variation in both composition and metabolomics of gut microbiota. (A) Relative Firmicutes/Bacteroidetes ratio in the cecum (n=6–12). (B) The relative abundance of bacteria at the phylum level in the cecum (n=6–12). (C) Scatter plots of unweighted uniFrac PCoA for the microbial composition in the cecal content (n=8). (D) Scatter plots of PCA for the microbioal metabolomics in the ceal content (n = 6). (E) Volcano plot of the metabolomic analysis (n=6). (F) PPD levels in cecal content and liver at ZT0 and ZT12 (n=5–6). The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05.
Figure 3:
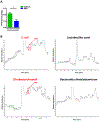
Production of PPD by human intestinal bacteria. (A) Cecal PPD level at ZT12 after antibiotics treatment at ZT12 (n=3). The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05. (B) Single bacteria strains were isolated from human stool and cultured in vitro. The PPD chromatograms (GC/MS) for the medium of specific bacteria strain.
Figure 4:
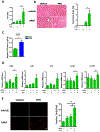
PPD synergistically enhanced APAP induced liver injury. Mice were treated with APAP or PPD at ZT0 for 24h. (A) Plasma ALT levels (n=5–9). (B) HE staining and necrotic area quantification (n=5–9). (C) Hepatic PPD level after PPD treatment (n=3). (D) mRNA levels of key cytokines and chemokines in the liver (n=5–9). (E) Primary cultured hepatocytes were treated with APAP, PPD or both for 6 hours, PI staining and quantification (n=3). The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05. Scale bar: 100 μm.
Figure 5:
PPD directly depleted GSH. (A) Mice were treated with or without ABX followed by APAP administration for 1h at ZT0 and ZT12, respectively. Hepatic p-JNK, JNK protein levels (n = 3). (B) Hepatic reduced GSH levels after antibiotics treatment (n=8–12). (C) Mice were treated with or without PPD for 30 min followed by APAP administration for 1h at ZT0. Hepatic p-JNK, JNK protein levels (n = 3). (D) Reduced GSH levels in the livers after PPD treatment for 1 hour at ZT0 (n=8–12). (E) Reduced GSH levels in primary hepatocyte after PPD treatment for 6 hours (n=8–12). (F) APAP adducts levels in the livers after PPD treatment for 1 hour at ZT0 (n = 3). The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05. N.S.: non-siginificance.
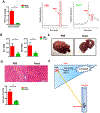
Figure 6:
The protective effect of Saccharomyces cerevisiae (Yeast) against APAP induced hepatotoxicity was accompanied with diminished PPD concentration in the cecum. Mice were gavaged with yeast at ZT10 for 3 days followed by APAP administration for 24h at ZT12. (A) Cecal PPD level and representative chromatograms (n=5–9). (B) Plasma ALT, AST levels (n=5–9). (C) Representative liver photography. (D) HE staining and necrotic area quantification (n=5–9). (E) Working model of this work: Gut microbiota generated more PPD at ZT12 and more PPD translocated into liver and synergistically enhanced hepaic damage induced by APAP through a mechanism that depended on PPD-induced GSH depletion, redox change or possibly other unknown mechanisms. The results are expressed as the mean ± S.E.M. Two-tailed Student’s t-test was used for statistical evaluation. *p<0.05. Scale bar: 100 μm.
Similar articles
- Effects of Photoperiod on Acetaminophen-Induced Hepatotoxicity in Mice.
Lu J, Wang H, Zhang R, Wan Z, Gao H, Cai J, Cheng Y, Pu D, Lin T, Fan C, Sun Y. Lu J, et al. Dig Dis Sci. 2020 Jan;65(1):178-188. doi: 10.1007/s10620-019-05749-6. Epub 2019 Aug 2. Dig Dis Sci. 2020. PMID: 31376085 - Gut Microbiota-Mediated Alterations of Hippocampal CB1R Regulating the Diurnal Variation of Cognitive Impairment Induced by Hepatic Ischemia-Reperfusion Injury in Mice.
He Z, Liu Y, Li Z, Sun T, Li Z, Liu C, Xiang H. He Z, et al. Neurochem Res. 2024 Aug;49(8):2165-2178. doi: 10.1007/s11064-024-04182-0. Epub 2024 Jun 2. Neurochem Res. 2024. PMID: 38824460 - The Discovery of Gut Microbial Metabolites as Modulators of Host Susceptibility to Acetaminophen-Induced Hepatotoxicity.
Lee H, Yang X, Jin PR, Won KJ, Kim CH, Jeong H. Lee H, et al. Drug Metab Dispos. 2024 Jul 16;52(8):754-764. doi: 10.1124/dmd.123.001541. Drug Metab Dispos. 2024. PMID: 38302428 Review. - Phenylpropionic acid produced by gut microbiota alleviates acetaminophen-induced hepatotoxicity.
Cho S, Yang X, Won KJ, Leone VA, Chang EB, Guzman G, Ko Y, Bae ON, Lee H, Jeong H. Cho S, et al. Gut Microbes. 2023 Jan-Dec;15(1):2231590. doi: 10.1080/19490976.2023.2231590. Gut Microbes. 2023. PMID: 37431867 Free PMC article. - Autophagy and acetaminophen-induced hepatotoxicity.
Shan S, Shen Z, Song F. Shan S, et al. Arch Toxicol. 2018 Jul;92(7):2153-2161. doi: 10.1007/s00204-018-2237-5. Epub 2018 Jun 6. Arch Toxicol. 2018. PMID: 29876591 Review.
Cited by
- Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice.
Gong S, Feng Y, Zeng Y, Zhang H, Pan M, He F, Wu R, Chen J, Lu J, Zhang S, Yuan S, Chen X. Gong S, et al. J Transl Med. 2021 Apr 13;19(1):147. doi: 10.1186/s12967-021-02814-5. J Transl Med. 2021. PMID: 33849559 Free PMC article. - Vitamin K1 ameliorates lipopolysaccharide-triggered skeletal muscle damage revealed by faecal bacteria transplantation.
Xiao Y, Feng J, Jia J, Li J, Zhou Y, Song Z, Guan F, Li X, Liu L. Xiao Y, et al. J Cachexia Sarcopenia Muscle. 2024 Feb;15(1):81-97. doi: 10.1002/jcsm.13379. Epub 2023 Nov 28. J Cachexia Sarcopenia Muscle. 2024. PMID: 38018317 Free PMC article. - Gut-Liver Axis as a Therapeutic Target for Drug-Induced Liver Injury.
Tao W, Fan Q, Wei J. Tao W, et al. Curr Issues Mol Biol. 2024 Feb 1;46(2):1219-1236. doi: 10.3390/cimb46020078. Curr Issues Mol Biol. 2024. PMID: 38392196 Free PMC article. Review. - [Gut microbiota-an important contributor to liver diseases].
Wang F, Cui Q, Zeng Y, Chen P. Wang F, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2020 Apr 30;40(4):595-600. doi: 10.12122/j.issn.1673-4254.2020.04.23. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32895142 Free PMC article. Chinese. - BTF3L4 Overexpression Mediates APAP-induced Liver Injury in Mouse and Cellular Models.
Lin J, Fan A, Yifu Z, Xie Q, Hong L, Zhou W. Lin J, et al. J Clin Transl Hepatol. 2024 Mar 28;12(3):245-256. doi: 10.14218/JCTH.2023.00342. Epub 2024 Feb 8. J Clin Transl Hepatol. 2024. PMID: 38426192 Free PMC article.
References
- Larsen FS, Wendon J. Understanding paracetamol-induced liver failure. Intensive Care Med 2014;40:888–890. - PubMed
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005;42:1364–1372. - PubMed
- Schäfer C, Schröder KR, Höglinger O, Tollabimazraehno S, Lornejad-Schäfer MR. Acetaminophen changes intestinal epithelial cell membrane properties, subsequently affecting absorption processes. Cell Physiol Biochem 2013;32(2):431–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical