A Mitochondrial Health Index Sensitive to Mood and Caregiving Stress - PubMed (original) (raw)
A Mitochondrial Health Index Sensitive to Mood and Caregiving Stress
Martin Picard et al. Biol Psychiatry. 2018.
Abstract
Background: Chronic life stress, such as the stress of caregiving, can promote pathophysiology, but the underlying cellular mechanisms are not well understood. Chronic stress may induce recalibrations in mitochondria leading to changes either in mitochondrial content per cell, or in mitochondrial functional capacity (i.e., quality).
Methods: Here we present a functional index of mitochondrial health (MHI) for human leukocytes that can distinguish between these two possibilities. The MHI integrates nuclear and mitochondrial DNA-encoded respiratory chain enzymatic activities and mitochondrial DNA copy number. We then use the MHI to test the hypothesis that daily emotional states and caregiving stress influence mitochondrial function by comparing healthy mothers of a child with an autism spectrum disorder (high-stress caregivers, n = 46) with mothers of a neurotypical child (control group, n = 45).
Results: The MHI outperformed individual mitochondrial function measures. Elevated positive mood at night was associated with higher MHI, and nightly positive mood was also a mediator of the association between caregiving and MHI. Moreover, MHI was correlated to positive mood on the days preceding, but not following the blood draw, suggesting for the first time in humans that mitochondria may respond to proximate emotional states within days. Correspondingly, the caregiver group, which had higher perceived stress and lower positive and greater negative daily affect, exhibited lower MHI. This effect was not explained by a mismatch between nuclear and mitochondrial genomes.
Conclusions: Daily mood and chronic caregiving stress are associated with mitochondrial functional capacity. Mitochondrial health may represent a nexus between psychological stress and health.
Keywords: Chronic stress; Daily affect; Mind-body; Mitochondria; Respiratory chain activity; mtDNA copy number.
Copyright © 2018 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
Figures
Figure 1
Mitochondrial health index (MHI) and mitochondrial profiling in human leukocytes. (A) Schematic of a human peripheral blood leukocyte and its mitochondria. The internal components of the mitochondrial respiratory chain (complexes I–V), the tricarboxilic acid ([TCA], also Krebs) cycle, and the mitochondrial genome (mtDNA) are shown in the inset with the inner mitochondrial membrane. (B) Mathematical integration of two nuclear DNA (nDNA)-encoded components (left), and mtDNA-related components (right) into the MHI. (C) Frequency distribution of MHI in the study sample. See Table 3 for statistics. (D) Correlation matrix generated by unsupervised clustering between enzymatic activities, calculated ratios, mtDNA copy number (mtDNAcn), and respiratory chain complexes protein levels. Three major clusters of correlated variables are highlighted. (E) Results from partial least square discriminant analysis model showing rank-ordered variables based on their variable importance in projection (VIP) scores for the first component of the full model; n = 89 to 91 for all. VIP values >1 are considered significant. ATP, adenosine triphosphate; COX, cytochrome c oxidase; CS, citrate synthase; Mito, mitochondrial; SDH, succinate dehydrogenase.
Figure 2
Exploratory analysis showing the strength of the association between daily emotional states and peripheral blood mononuclear cells’ mitochondrial health index (MHI). (A) Combined effect size for the association between MHI and emotional states measured 1) across the week: Week average; 2) over 3 days preceding peripheral blood mononuclear cell collection: Days before; or 3) over 3 days after peripheral blood mononuclear cell collection: Days after. Note that effect sizes are larger for time points preceding blood draw, suggesting a directional relationship from mood to mitochondria. Mood was assessed in the morning and at night as described in the Supplemental Methods and Materials. +p < .10; *p < .05, **p < .01; n = 86 to 89. N.S., not significant. (B) Individual Pearson’s r correlation coefficients between daily measures of positive or negative mood and MHI measured from blood drawn at day 4; n = 86 to 89.
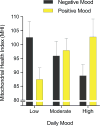
Figure 3
Cross-sectional association between mood and mitochondrial health index (MHI). Average MHI for tertiles of nightly negative and positive mood across the 3 days preceding mitochondrial measurements. Data are mean ± SEM; n = 27 to 29 per tertile.
Similar articles
- Mitochondrial health index correlates with plasma circulating cell-free mitochondrial DNA in bipolar disorder.
Cordeiro RC, Lima CNC, Fries GR, Zunta-Soares G, Soares JC, Quevedo J, Scaini G. Cordeiro RC, et al. Mol Psychiatry. 2023 Nov;28(11):4622-4631. doi: 10.1038/s41380-023-02249-y. Epub 2023 Sep 15. Mol Psychiatry. 2023. PMID: 37723283 - Failed upregulation of TFAM protein and mitochondrial DNA in oxidatively deficient fibers of chronic obstructive pulmonary disease locomotor muscle.
Konokhova Y, Spendiff S, Jagoe RT, Aare S, Kapchinsky S, MacMillan NJ, Rozakis P, Picard M, Aubertin-Leheudre M, Pion CH, Bourbeau J, Hepple RT, Taivassalo T. Konokhova Y, et al. Skelet Muscle. 2016 Feb 18;6:10. doi: 10.1186/s13395-016-0083-9. eCollection 2016. Skelet Muscle. 2016. PMID: 26893822 Free PMC article. - The health and well-being of caregivers of children with cerebral palsy.
Raina P, O'Donnell M, Rosenbaum P, Brehaut J, Walter SD, Russell D, Swinton M, Zhu B, Wood E. Raina P, et al. Pediatrics. 2005 Jun;115(6):e626-36. doi: 10.1542/peds.2004-1689. Pediatrics. 2005. PMID: 15930188 - Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders.
Morella IM, Brambilla R, Morè L. Morella IM, et al. Neurosci Biobehav Rev. 2022 Nov;142:104892. doi: 10.1016/j.neubiorev.2022.104892. Epub 2022 Sep 28. Neurosci Biobehav Rev. 2022. PMID: 36181925 Review. - The mitochondrial electron transport and oxidative phosphorylation system.
Hatefi Y. Hatefi Y. Annu Rev Biochem. 1985;54:1015-69. doi: 10.1146/annurev.bi.54.070185.005055. Annu Rev Biochem. 1985. PMID: 2862839 Review. No abstract available.
Cited by
- Mitochondrial signal transduction.
Picard M, Shirihai OS. Picard M, et al. Cell Metab. 2022 Nov 1;34(11):1620-1653. doi: 10.1016/j.cmet.2022.10.008. Cell Metab. 2022. PMID: 36323233 Free PMC article. Review. - To promote healthy aging, focus on the environment.
Belsky DW, Baccarelli AA. Belsky DW, et al. Nat Aging. 2023 Nov;3(11):1334-1344. doi: 10.1038/s43587-023-00518-7. Epub 2023 Nov 9. Nat Aging. 2023. PMID: 37946045 Review. - Psychological and biological mechanisms linking trauma with cardiovascular disease risk.
Sumner JA, Cleveland S, Chen T, Gradus JL. Sumner JA, et al. Transl Psychiatry. 2023 Jan 27;13(1):25. doi: 10.1038/s41398-023-02330-8. Transl Psychiatry. 2023. PMID: 36707505 Free PMC article. Review. - Stress and Immunological Aging.
Reed RG. Reed RG. Curr Opin Behav Sci. 2019 Aug;28:38-43. doi: 10.1016/j.cobeha.2019.01.012. Epub 2019 Feb 26. Curr Opin Behav Sci. 2019. PMID: 31179376 Free PMC article. - Individual physiological and mitochondrial responses during 12 weeks of intensified exercise.
Jacques M, Landen S, Alvarez Romero J, Yan X, Garnham A, Hiam D, Siegwald M, Mercier E, Hecksteden A, Eynon N, Voisin S. Jacques M, et al. Physiol Rep. 2021 Aug;9(15):e14962. doi: 10.14814/phy2.14962. Physiol Rep. 2021. PMID: 34327858 Free PMC article.
References
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. - PubMed
- Steptoe A, Kivimaki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical