Obesity, Metabolic Syndrome, and Musculoskeletal Disease: Common Inflammatory Pathways Suggest a Central Role for Loss of Muscle Integrity - PubMed (original) (raw)
Review
Obesity, Metabolic Syndrome, and Musculoskeletal Disease: Common Inflammatory Pathways Suggest a Central Role for Loss of Muscle Integrity
Kelsey H Collins et al. Front Physiol. 2018.
Abstract
Inflammation can arise in response to a variety of stimuli, including infectious agents, tissue injury, autoimmune diseases, and obesity. Some of these responses are acute and resolve, while others become chronic and exert a sustained impact on the host, systemically, or locally. Obesity is now recognized as a chronic low-grade, systemic inflammatory state that predisposes to other chronic conditions including metabolic syndrome (MetS). Although obesity has received considerable attention regarding its pathophysiological link to chronic cardiovascular conditions and type 2 diabetes, the musculoskeletal (MSK) complications (i.e., muscle, bone, tendon, and joints) that result from obesity-associated metabolic disturbances are less frequently interrogated. As musculoskeletal diseases can lead to the worsening of MetS, this underscores the imminent need to understand the cause and effect relations between the two, and the convergence between inflammatory pathways that contribute to MSK damage. Muscle mass is a key predictor of longevity in older adults, and obesity-induced sarcopenia is a significant risk factor for adverse health outcomes. Muscle is highly plastic, undergoes regular remodeling, and is responsible for the majority of total body glucose utilization, which when impaired leads to insulin resistance. Furthermore, impaired muscle integrity, defined as persistent muscle loss, intramuscular lipid accumulation, or connective tissue deposition, is a hallmark of metabolic dysfunction. In fact, many common inflammatory pathways have been implicated in the pathogenesis of the interrelated tissues of the musculoskeletal system (e.g., tendinopathy, osteoporosis, and osteoarthritis). Despite these similarities, these diseases are rarely evaluated in a comprehensive manner. The aim of this review is to summarize the common pathways that lead to musculoskeletal damage and disease that result from and contribute to MetS. We propose the overarching hypothesis that there is a central role for muscle damage with chronic exposure to an obesity-inducing diet. The inflammatory consequence of diet and muscle dysregulation can result in dysregulated tissue repair and an imbalance toward negative adaptation, resulting in regulatory failure and other musculoskeletal tissue damage. The commonalities support the conclusion that musculoskeletal pathology with MetS should be evaluated in a comprehensive and integrated manner to understand risk for other MSK-related conditions. Implications for conservative management strategies to regulate MetS are discussed, as are future research opportunities.
Keywords: MAPK; NFkB; bone; joint diseases; muscle; tendon.
Figures
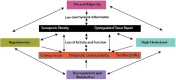
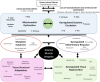
Figure 1
The interface between metabolic complications and musculoskeletal compromise.
Figure 2
Potential impact of changes in muscle damage on lower limb motion segment integrity.
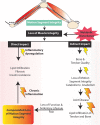
Figure 3
Structural and inflammatory changes in muscle with obesity; (A) factors that influence muscle structural integrity with metabolic challenge; (B) alterations in adipose tissue; (C) musculoskeletal consequences of chronic-low grade inflammation.
Figure 4
Vulnerability and protection of muscle with diet-induced obesity may be determined by oxidative capacity; (A) system level changes; (B) tissue-level changes; (C) cellular and molecular level alterations.
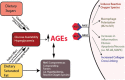
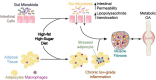
Figure 5
Interacting variables reinforcing metabolic dysfunction and sequelae. Links between dietary sugar, dietary saturated fat, increased hyperglycaemia, advanced glycation end products (AGEs), their receptors (RAGEs), and inflammation, macrophage polarization, and collagen cross-linking.
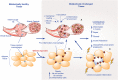
Figure 6
Markers of muscle integrity are associated with metabolic OA severity after 12-weeks of high-fat high-sucrose diet-induced obesity. (ORO, Oil Red O stain for intramuscular fat; Picro, Picrosirius stain for collagen).
Figure 7
Overview of tissue adaptations and processes with diet-induced obesity.
Figure 8
Systemic mediators and compromised muscle integrity are associated with Metabolic OA onset and progression, adapted from Collins et al. (2015a).
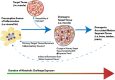
Figure 9
Conceptual framework for inflammatory initiation by a presumptive inflammatory source (i.e., visceral fat, gut microbiota), initiation of adaptation, and subsequent damage in primary target tissue. There is likely a “ripple effect” to the motion segment tissues. Whether associated motion segment tissues becomie inflammatory sources, affecting a presumptive inflammatory source, as well as whether damage is reversible in these tissues, represent interesting open research questions in this area.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials