Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway - PubMed (original) (raw)
doi: 10.1186/s13046-018-0677-7.
Mengjie Liu 1, Shengyang Qu 1, Jiequn Ma 1, Yamin Zhang 1, Tingting Shi 1, Hongqing Wen 1 2, Yujuan Yang 3, Shuhong Wang 1, Jing Wang 1, Kejun Nan 1, Yu Yao 4, Tao Tian 5
Affiliations
- PMID: 29530052
- PMCID: PMC5846230
- DOI: 10.1186/s13046-018-0677-7
Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway
Xiao Fu et al. J Exp Clin Cancer Res. 2018.
Retraction in
- Retraction Note: Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway.
Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, Nan K, Yao Y, Tian T. Fu X, et al. J Exp Clin Cancer Res. 2023 Jul 19;42(1):174. doi: 10.1186/s13046-023-02761-7. J Exp Clin Cancer Res. 2023. PMID: 37464377 Free PMC article. No abstract available.
Abstract
Background: Multidrug resistance is the main obstacle for hepatocellular carcinoma (HCC) treatment. miR-32-5p is involved in HCC progression but its function in multidrug resistance is still unclear. Here we aim to find out the function of miR-32-5p in inducing multidrug resistance and its underlying mechanisms of transforming sensitive cell to resistant cell.
Methods: We detected the expression of miR-32-5p and PTEN in the multidrug-resistant cell line (Bel/5-FU) and the sensitive cell line (Bel7402), HCC and para-carcinoma liver tissues through real-time PCR. Dual-luciferase reporter assay verified PTEN is the target of miR-32-5p. Exosomes from sensitive and multidrug resistant cell line were obtained and confirmed through ultracentrifuge and Nano Analyzer. Gain- and loss-of-function experiments, rescue experiments, a PI3K/Akt pathway inhibitor, an exosome biogenesis inhibitor, and nude mice xenograft models were used to determine the underlying mechanisms of miR-32-5p and PTEN, as well as exosomal miR-32-5p in inducing multidrug resistance in vitro and in vivo.
Results: miR-32-5p was significantly elevated but PTEN was reduced in Bel/5-FU. An inverse correlation between miR-32-5p and PTEN was confirmed in HCC cell lines and patients; moreover, high expression of miR-32-5p and low expression of PTEN were positively associated with poor prognosis. Over-expression of miR-32-5p activated the PI3K/Akt pathway by suppressing PTEN and induced multidrug resistance via exosomes through promoting angiogenesis and epithelial-mesenchymal transition (EMT).
Conclusions: Our study demonstrated that the multidrug-resistant cell, Bel/5-FU delivers miR-32-5p to sensitive cell, Bel7402 by exosomes and activates the PI3K/Akt pathway to further induce multidrug resistance by modulating angiogenesis and EMT.
Keywords: Exosome; Hepatocellular carcinoma; Multidrug resistance; PTEN; microRNA-32-5p.
Conflict of interest statement
Ethics approval and consent to participate
All protocols were approved by the Ethics Committee of Xi’an Jiaotong University, and informed consent was obtained from all patients before surgery. And all in vivo protocols were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest were disclosed.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
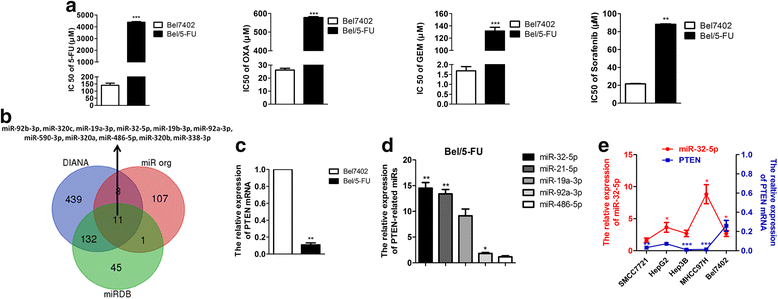
MiR-32-5p induces multidrug-resistance in HCC. a Bel/5-FU is resistant to 5-FU, OXA, GEM and sorafenib, n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. 5-FU, 5-fluorouracil; OXA, oxaliplatin; GEM, gemcitabine. b Predicted miRs that have complementary binding sites to the 3’-UTR of PTEN through bioinformatics analysis (DIANA, miRDB, and miR org databases). c PTEN mRNA is lower expressed in Bel/5-FU. The PTEN level is normalized to the corresponding inner control GAPDH, and the PTEN/GAPDH in Bel7402 cells is set as 1.0-fold, n = three independent experiments, **p < 0.01 by Student’s _t_-test. d The expression of PTEN-related miRs in Bel/5-FU. The miRs levels are normalized to the corresponding inner control U6, and the miRs/U6 in Bel7402 cells is set as 1.0-fold, n = three independent experiments, **p < 0.01 by Student’s _t_-test; miR, microRNA. e The reversed expression pattern of miR-32-5p and PTEN in HCC cell lines, n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA test
Fig. 2
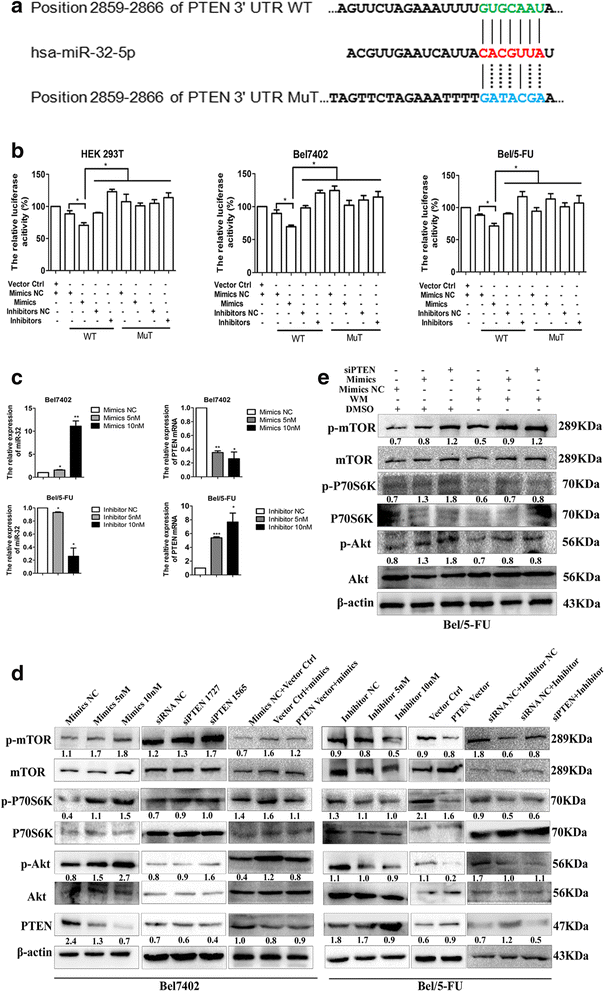
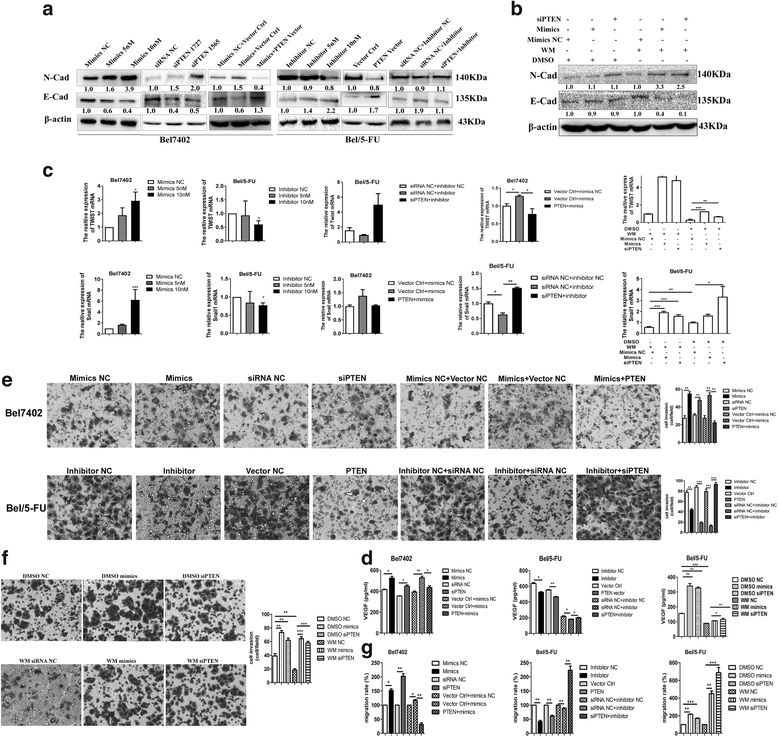
miR-32-5p targets PTEN and activates the PI3K/Akt pathway. a miR-32-5p and its putative binding sites in the 3’-UTR of PTEN. Mutant miR-32-5p binding sites were generated in the complementary site for the seed region of miR-32-5p. b Effects of miR-32-5p on luciferase activity in Bel7402, Bel/5-FU, and HEK293T cells carrying the WT and MuT 3’-UTR of PTEN. n = three independent experiments, *p < 0.05 by Student’s _t_-test. c The expression of miR-32-5p and PTEN mRNA according to the increasing dose of miR-32-5p mimics and inhibitor by real-time PCR. The miR-32-5p level is normalized to the corresponding inner control U6, the PTEN level is calculated using the corresponding internal control GAPDH and the miRs/U6 or PTEN/GAPDH in Bel7402 cells is set as 1.0-fold. n = three independent experiments, *p < 0.05, **p < 0.01 by Student’s _t_-test. d, e The expression of PTEN, phosphorylation of Akt, P70S6K, and mTOR according to the dose of miR-32-5p mimics and inhibitor by Western blots; the up- or down-regulation of PTEN replicates the effects of miR-32-5p inhibitor or mimics, respectively, and PTEN-expressing vector or siPTEN reverses the expression of PTEN and phosphorylation of Akt, P70S6K and mTOR in the cells transfected miR-32-5p mimics or inhibitor, respectively. miR-32-5p mimics and PTEN-targeting siRNA rescue the expression of p-Akt, p-P70S6K and p-mTOR after WM treatment. The relative expression of PTEN is normalized to the level of the corresponding internal control β-actin, and the relative expression of p-Akt, p-P70S6K and p-mTOR is normalized to the levels of Akt, P70S6K, and mTOR, respectively. WT, wild-type; MuT, mutant; p-Akt, phosphorylated Akt; p-P70S6K, phosphorylated P70S6K; p-mTOR, phosphorylated mTOR; siPTEN, PTEN siRNA; WM, Wortmannin
Fig. 3
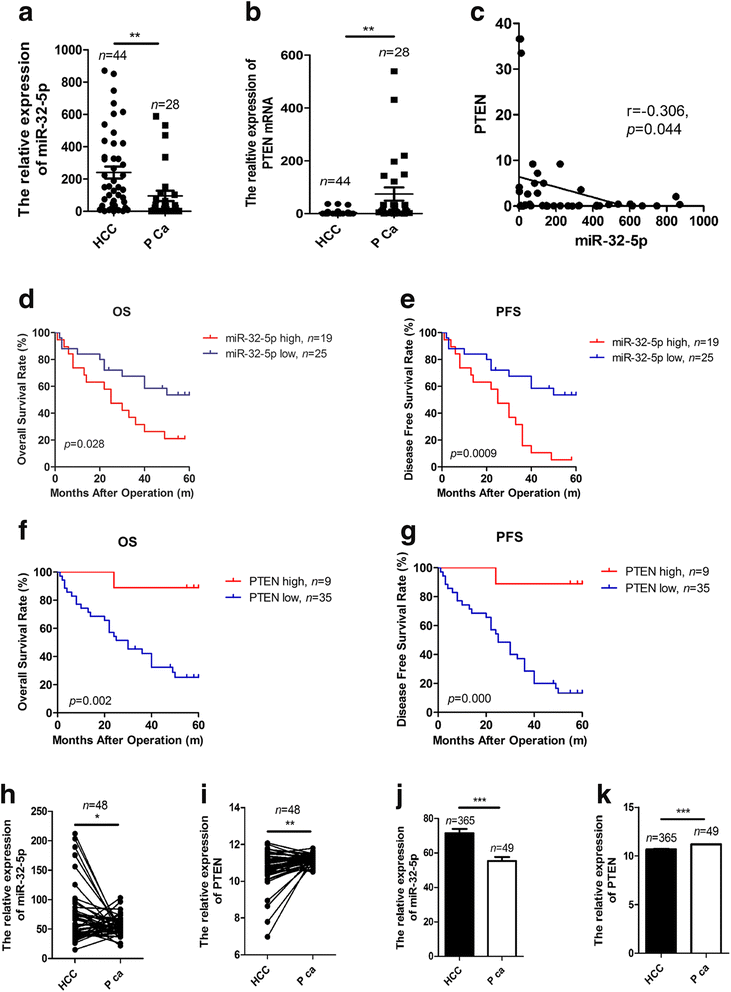
Reversed correlations of miR-32-5p and PTEN. a, b miR-32-5p is up-regulated but PTEN is down-regulated in 44 HCC specimens, compared with 28 unpaired para-carcinoma liver tissues. P Ca, paracarcinoma liver tissues. The miR-32-5p and PTEN levels were calculated using the corresponding inner control U6 and GAPDH respectively, **p < 0.01 by non-parametric Mann-Whitney-Wilcoxon test. c Reversed correlations of miR-32-5p and PTEN in HCC patients’ tissues, by Pearson’s correlation test. d-g High miR-32-5p or low PTEN in 44 HCC patients predicts shorter OS and PFS, by Kaplan-Meier analysis. OS, overall survival, PFS, progression-free survival. h-k miR-32-5p is up-regulated but PTEN is down-regulated in both paired and un-paired HCC and paracarcinoma liver specimens in TCGA dataset. *p < 0.05, **p < 0.01, ***p < 0.001 by non-parametric Mann-Whitney-Wilcoxon test
Fig. 4
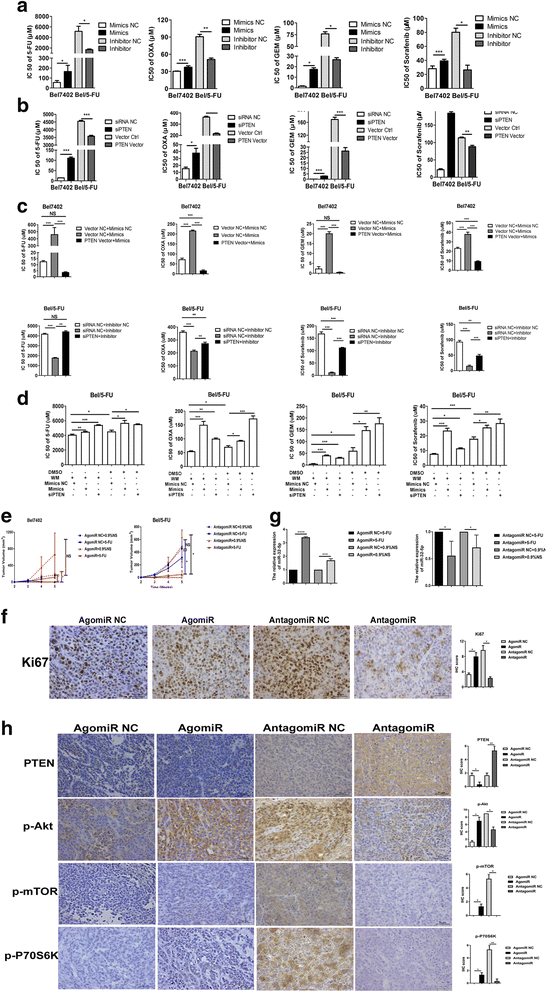
miR-32-5p promotes multidrug resistance. a-d Elevated or reduced expression of miR-32-5p induces or inhibits resistance to 5-FU, OXA, GEM, and sorafenib in vitro. siPTEN enhances, whereas PTEN-expressing vector reverses the resistance to 5-FU, OXA, GEM, and sorafenib. Ectopic expression of PTEN in Bel7402 cells transfected with miR-32-5p mimics rescues the resistance to 5-FU, OXA, GEM, and sorafenib, while inhibition of PTEN in Bel/5-FU cells transduced with miR-32-5p inhibitor reverses the inhibition of 5-FU, OXA, GEM, and sorafenib. WM sensitizes Bel/5-FU cells to 5-FU, OXA, GEM, and sorafenib, but miR-32-5p mimics or siPTEN increases multidrug resistance. n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test or one-way ANOVA test. e Growth curves of xenograft tumors derived from Bel7402 cells injected with agomiR and Bel/5-FU cells injected with antagomiR in response to 0.9%NS or 5-FU. *p < 0.05, by one-way ANOVA test. 0.9%NS, 0.9% normal saline. f, h IHC staining for Ki67, PTEN, p-Akt, p-mTOR, and p-P70S6K in xenograft tumors; original magnification, 400×; scale bar, 25 μm. p-Akt, phosphorylated Akt; p-P70S6K, phosphorylated P70S6K; p-mTOR, phosphorylated mTOR. g The expression of miR-32-5p in xenograft tumors by real-time PCR. The expression of miR-32-5p is normalized to the level of the corresponding internal control U6. *p < 0.05, **p < 0.01 by Student’s _t_-test
Fig. 5
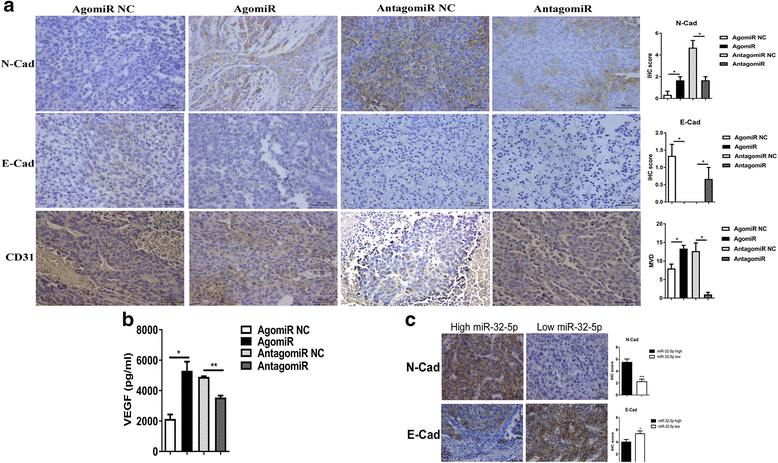
miR-32-5p promotes multidrug resistance through EMT and angiogenesis in vivo. a IHC staining for N-Cad, E-Cad, and CD31 in xenograft tumors. Original magnification, 400×; scale bar, 25 μm. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. N-Cad, N-Cadherin; E-Cad, E-Cadherin; MVD, microvascular density. b The expression of VEGF in xenograft tumors. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. c IHC staining for N-Cad and E-Cad in HCC patients’ tissues. Original magnification, 400×; scale bar, 25 μm. *p < 0.05, **p < 0.01, ***p < 0.001 by non-parametric Mann-Whitney-Wilcoxon test
Fig. 6
miR-32-5p promotes multidrug resistance through angiogenesis and EMT in vitro. (a-b) Western blots for the expression of N-Cad and E-Cad in Bel7402 and Bel/5-FU cell lines transfected with miR-32-5p mimics, inhibitor, siPTEN, or PTEN-expressing vector; co-transfected with miR-32-5p mimics and PTEN-expressing vector or miR-32-5p inhibitor and siPTEN; WM-treated Bel/5-FU cell lines transfected with miR-32-5p mimics, siPTEN, or respective NC. The expression of N-Cad and E-Cad are normalized to the level of the corresponding internal control β-actin. c Real-time PCR to analyze the expression of Twist and Snail mRNA in Bel7402 and Bel/5-FU cell lines transfected with miR-32-5p mimics, inhibitor, siPTEN or PTEN-expressing vector; co-transfected with miR-32-5p mimics and PTEN-expressing vector or miR-32-5p inhibitor and siPTEN; and WM-treated Bel/5-FU cell lines transfected miR-32-5p mimics or siPTEN. The levels of Twist and Snail mRNA are normalized to the level of the corresponding internal control GAPDH. n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA test. d ELISA for VEGF in the supernatant of Bel7402 and Bel/5-FU cell lines transfected with miR-32-5p mimics, inhibitor, siPTEN or PTEN-expressing vector, co-transfected with miR-32-5p mimics and PTEN-expressing vctor or miR-32-5p inhibitor and siPTEN, and WM-treated Bel/5-FU cell lines transfected with miR-32-5p mimics or siPTEN. n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. e-g Transwell and wound healing assays in Bel7402 and Bel/5-FU cell lines transfected with miR-32-5p mimics, inhibitor, siPTEN or PTEN-expressing vector, co-transfected with miR-32-5p mimics and PTEN-expressing vector or miR-32-5p inhibitor and siPTEN, and WM-treated Bel/5-FU cell lines transfected with miR-32-5p mimics or siPTEN. n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test
Fig. 7
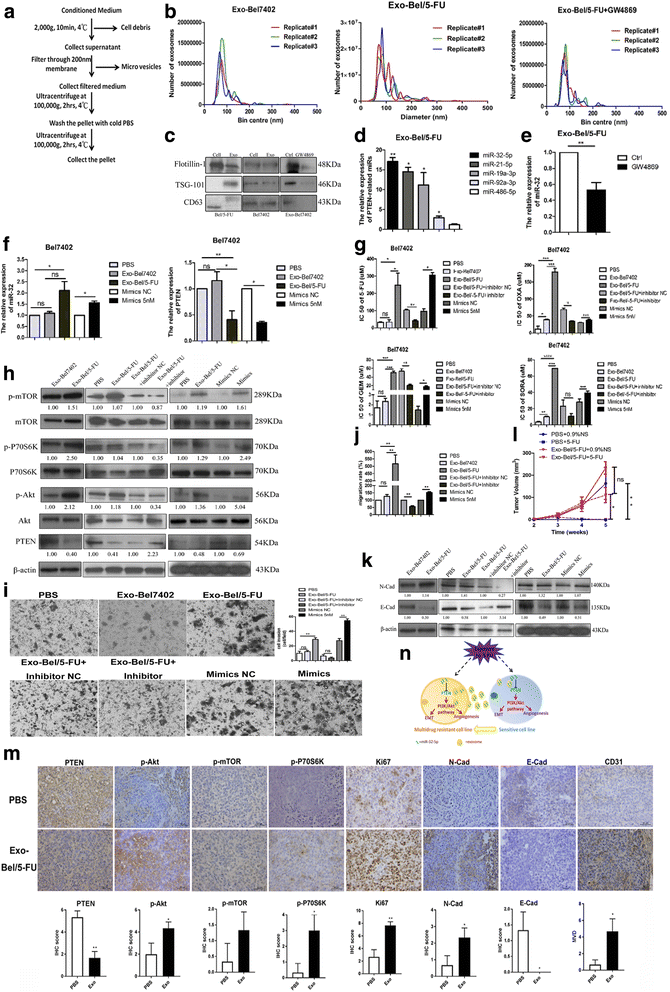
Exosomal miR-32-5p induces multidrug resistance in vitro and in vivo. a Schematic diagram of exosome extraction. b The sizes and numbers of exosomes were determined by a Delsa Nano Analyzer. c Western blots for the expression of the exosome markers Flotillin-1, TSG-101, and CD63. d miR-32-5p was overexpressed in exosomes from Bel/5-FU. e Real-time PCR for the expression of miR-32-5p in exosomes treated with ethanol and GW-4869. f Real-time PCR for the expression of miR-32-5p and PTEN in Bel7402 cells treated with PBS, exosomes from Bel/5-FU, mimics NC and mimics. g Exosomes from Bel/5-FU but not from Bel7402 induced multidrug resistance, and miR-32-5p inhibitor reversed resistance in Bel7402 cells. (h and k) Western blots for the expression of molecules of the PI3K/Akt pathway, E-cad and N-Cad in Bel7402 cells after treatment with PBS, exosomes from Bel7402 and Bel/5-FU; in Bel7402 cells into which miR-32-5p inhibitor or inhibitor NC was transferred after treatment with exosomes; and in Bel7402 cells transfected with miR-32-5p mimics and the respective NC. (i and j) Migration and invasion assay of Bel7402 cells after treatment with PBS, exosomes from Bel7402 and Bel/5-FU; Bel7402 cells into which miR-32-5p inhibitor or inhibitor NC was transferred after treatment with exosomes; and Bel7402 transfected with miR-32-5p mimics and the respective NC. (l) Growth curves of xenograft tumors derived from Bel7402 cells injected with exosomes and then treated with PBS or 5-FU. n = three independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. (m) IHC staining for PTEN, p-Akt, p-mTOR, p-P70S6K, Ki67, N-Cad, E-Cad, and CD31 in xenograft tumors. Original magnification, 400×; scale bar, 25 μm. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s _t_-test. N-Cad, N-Cadherin; E-Cad, E-Cadherin; MVD, microvascular density. (n) Schematic diagram summarizing how exosomal miR-32-5p induces multidrug resistance via the PTEN/PI3K/Akt pathway through angiogenesis and EMT. Exo, exosome
Similar articles
- MicroRNA-155-5p promotes hepatocellular carcinoma progression by suppressing PTEN through the PI3K/Akt pathway.
Fu X, Wen H, Jing L, Yang Y, Wang W, Liang X, Nan K, Yao Y, Tian T. Fu X, et al. Cancer Sci. 2017 Apr;108(4):620-631. doi: 10.1111/cas.13177. Epub 2017 Apr 19. Cancer Sci. 2017. PMID: 28132399 Free PMC article. - DEAD-Box Helicase 17 circRNA (circDDX17) Reduces Sorafenib Resistance and Tumorigenesis in Hepatocellular Carcinoma.
Zhang X, Wang W, Mo S, Sun X. Zhang X, et al. Dig Dis Sci. 2024 Jun;69(6):2096-2108. doi: 10.1007/s10620-024-08401-0. Epub 2024 Apr 23. Dig Dis Sci. 2024. PMID: 38653946 - Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway.
Li WF, Dai H, Ou Q, Zuo GQ, Liu CA. Li WF, et al. Tumour Biol. 2016 May;37(5):5885-95. doi: 10.1007/s13277-015-4456-1. Epub 2015 Nov 21. Tumour Biol. 2016. PMID: 26589417 - Growing Evidence of Exosomal MicroRNA-Related Metastasis of Hepatocellular Carcinoma.
Sun W, Fu S, Wu S, Tu R. Sun W, et al. Biomed Res Int. 2020 Nov 27;2020:4501454. doi: 10.1155/2020/4501454. eCollection 2020. Biomed Res Int. 2020. PMID: 33313314 Free PMC article. Review. - The Role of the MiR-181 Family in Hepatocellular Carcinoma.
Chen J, Liu K, Vadas MA, Gamble JR, McCaughan GW. Chen J, et al. Cells. 2024 Jul 31;13(15):1289. doi: 10.3390/cells13151289. Cells. 2024. PMID: 39120319 Free PMC article. Review.
Cited by
- H-TEX-mediated signaling between hepatocellular carcinoma cells and macrophages and exosome-targeted therapy for hepatocellular carcinoma.
Yu S, Zhou L, Fu J, Xu L, Liu B, Zhao Y, Wang J, Yan X, Su J. Yu S, et al. Front Immunol. 2022 Oct 13;13:997726. doi: 10.3389/fimmu.2022.997726. eCollection 2022. Front Immunol. 2022. PMID: 36311698 Free PMC article. Review. - Multifaceted roles of aerobic glycolysis and oxidative phosphorylation in hepatocellular carcinoma.
Zhang Y, Li W, Bian Y, Li Y, Cong L. Zhang Y, et al. PeerJ. 2023 Feb 1;11:e14797. doi: 10.7717/peerj.14797. eCollection 2023. PeerJ. 2023. PMID: 36748090 Free PMC article. Review. - MiR-32-5p aggravates intestinal epithelial cell injury in pediatric enteritis induced by Helicobacter pylori.
Feng J, Guo J, Wang JP, Chai BF. Feng J, et al. World J Gastroenterol. 2019 Nov 7;25(41):6222-6237. doi: 10.3748/wjg.v25.i41.6222. World J Gastroenterol. 2019. PMID: 31749593 Free PMC article. - Drug resistance mechanism of kinase inhibitors in the treatment of hepatocellular carcinoma.
Jiang L, Li L, Liu Y, Lu L, Zhan M, Yuan S, Liu Y. Jiang L, et al. Front Pharmacol. 2023 Feb 13;14:1097277. doi: 10.3389/fphar.2023.1097277. eCollection 2023. Front Pharmacol. 2023. PMID: 36891274 Free PMC article. Review. - The role of extracellular vesicles in acquisition of resistance to therapy in glioblastomas.
Yekula A, Taylor A, Beecroft A, Kang KM, Small JL, Muralidharan K, Rosh Z, Carter BS, Balaj L. Yekula A, et al. Cancer Drug Resist. 2021 Mar 19;4(1):1-16. doi: 10.20517/cdr.2020.61. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582008 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 81301909/National Natural Science Foundation of China
- 81672810/National Natural Science Foundation of China
- 2016KW-017/International Cooperation Project in Science and Technology of Shaanxi Province
- 2017JM8019/National Science Foundation of Shaanxi Province
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials