Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System - PubMed (original) (raw)
Review
Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System
Shenglong Zou et al. Int J Mol Sci. 2018.
Abstract
The biological effects of cannabinoids, the major constituents of the ancient medicinal plant Cannabis sativa (marijuana) are mediated by two members of the G-protein coupled receptor family, cannabinoid receptors 1 (CB1R) and 2. The CB1R is the prominent subtype in the central nervous system (CNS) and has drawn great attention as a potential therapeutic avenue in several pathological conditions, including neuropsychological disorders and neurodegenerative diseases. Furthermore, cannabinoids also modulate signal transduction pathways and exert profound effects at peripheral sites. Although cannabinoids have therapeutic potential, their psychoactive effects have largely limited their use in clinical practice. In this review, we briefly summarized our knowledge of cannabinoids and the endocannabinoid system, focusing on the CB1R and the CNS, with emphasis on recent breakthroughs in the field. We aim to define several potential roles of cannabinoid receptors in the modulation of signaling pathways and in association with several pathophysiological conditions. We believe that the therapeutic significance of cannabinoids is masked by the adverse effects and here alternative strategies are discussed to take therapeutic advantage of cannabinoids.
Keywords: cannabinoid; central nervous system; endocannabinoid; receptor; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
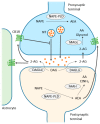
Figure 1
Simplified scheme representing endocannabinoid retrograde signaling mediated synaptic transmission. Endocannabinoids are produced from postsynaptic terminals upon neuronal activation. As the two major endocannabinoids shown in the scheme, 2-arachidonolglycerol (2-AG) is biosynthesized from diacylglycerol (DAG) by diacylglycerol lipase-α (DAGLα), and anandamide (AEA) is synthesized from _N_-acyl-phosphatidylethanolamine (NAPE) by NAPE-specific phospholipase D (NAPE-PLD). As lipids, endocannabinoids, mainly 2-AG, readily cross the membrane and travel in a retrograde fashion to activate CB1Rs located in the presynaptic terminals. Activated CB1Rs will then inhibit neurotransmitter (NT) release through the suppression of calcium influx. 2-AG is also able to activate CB1Rs located in astrocytes, leading to the release of glutamate. Extra 2-AG in the synaptic cleft is taken up into the presynaptic terminals, via a yet unclear mechanism, and degraded to arachidonic acid (AA) and glycerol by monoacylglycerol lipase (MAGL). On the other hand, AEA, synthesized in postsynaptic terminal, activates intracellular CB1R and other non-CBR targets, such as the transient receptor potential cation channel subfamily V member 1 (TRPV1). Although endocannabinoid retrograde signaling is mainly mediated by 2-AG, AEA can activate presynaptic CB1Rs as well. Fatty acid amide hydrolase (FAAH) is primarily found in postsynaptic terminals and is responsible for degrading AEA to AA and ethanolamine (EtNH2). Although NAPE-PLD is expressed in presynaptic terminals in several brain regions, it is not clear yet whether AEA is responsible for anterograde signaling in the endocannabinoid system. Note that alternative routes exist for the metabolism of endocannabinoids, depending on the brain region and physiological conditions. Thin arrows indicate enzymatic process; thick arrows indicate translocation; blunted arrow indicates inhibition.
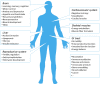
Figure 2
Major localization sites and associated functions of the CB1R in the human body. The majority of CB1Rs expressed in human body is found in the brain, where it is involved in various neurological activities. CB1Rs on the peripheral sites, although to a lesser extent, participates in the regulation of local tissue functions.
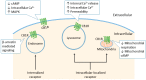
Figure 3
Subcellular localization of the CB1R. Typically, the CB1R is located at cell surface and inhibits cyclic adenosine monophosphate (cAMP) formation and calcium influx upon activation. Constitutive and ligand-induced internalized CB1Rs mediate signaling pathways through β-arrestin. Intracellular-localized CB1Rs do not translocate to plasma membrane. Instead, they form a subpopulation with pharmacological properties distinct from their plasma membrane-localized counterparts. CB1Rs located on lysosomes can increase intracellular calcium concentrations through the release of internal calcium stores, and increase the permeability of lysosomes. Mitochondrial CB1Rs inhibit mitochondrial cellular respiration and cAMP production, hence regulating cellular energy metabolism.
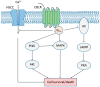
Figure 4
CB1R-modulated major signaling pathways. Typically, the CB1R is coupled to Gi/o and inhibits the activity of adenylyl cyclase (AC), formation of cyclic adenosine monophosphate (cAMP), and the activity of protein kinase A (PKA). Under certain circumstances, the CB1R can switch its coupling of G protein from Gi/o to Gs or Gq. The CB1R is able to suppress calcium influx via voltage-gated calcium channel (VGCC). Several mitogen-activated protein kinases (MAPKs), including ERK1/2, p38, and JNK, are activated by the CB1R. The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway is activated by CB1R as well. Depending on the ligand and subcellular environment, the outcome of CB1R-mediated signaling could be promotion of cell survival or cell death. Arrows indicate stimulation; blunted arrows indicate inhibition.
References
- Mechoulam R. The Pharmacohistory of Cannabis sativa, in Cannabis as Therapeutic Agent. CRC Press; Boca Raton, FL, USA: 1986.
- Iversen L. The Science of Marijuana. Oxford University Press; Oxford, UK: 2000.
- Gaoni Y., Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical