An intracrine view of sex steroids, immunity, and metabolic regulation - PubMed (original) (raw)
Review
An intracrine view of sex steroids, immunity, and metabolic regulation
Katya B Rubinow. Mol Metab. 2018 Sep.
Abstract
Background: Over the past two decades, parallel recognition has grown of the importance of both sex steroids and immune activity in metabolic regulation. More recently, these discrete areas have been integrated in studies examining the metabolic effects of sex steroid immunomodulation. Implicit in these studies has been a traditional, endocrine model of sex steroid delivery from the gonads to target cells, including immune cells. Thus, research to date has focused on the metabolic effects of sex steroid receptor signaling in immune cells. This endocrine model, however, overlooks the extensive capacity of immune cells to generate and metabolize sex steroids, enabling the production of sex steroids for intracrine signaling - that is, sex steroid production for signaling within the cell of origin. Intracrine function allows highly cell-autonomous regulation of sex steroid exposure, and sex steroid secretion by immune cells could confer paracrine signaling effects in neighboring cells within metabolic tissues. In this review, immune cell intracrinology will denote sex steroid production within immune cells for either intracrine or paracrine signaling. This intracrine capacity of immune cells has been well established, and prior work has supported its importance in autoimmune disorders, trauma, and cancer. The potential relevance of immune cell intracrine function to the regulation of energy balance, body weight, body composition, and insulin sensitivity has yet to be explored.
Scope of review: The following review will detail findings to date regarding the steroidogenic and steroid metabolizing capacity of immune cells, the regulation of immune cell intracrine function, and the biological effects of immune-derived sex steroids, including the clinical relevance of immune cell intracrinology in fields other than metabolism. These findings will serve as the basis for a proposed model of immune cell intracrinology constituting a new frontier in metabolism research.
Major conclusions: The development of highly sensitive mass spectrometric methods for sex steroid measurement and quantitation of metabolic flux now allows unprecedented ability to interrogate sex steroid production, metabolism and secretion by immune cells. Immune cell intracrinology could reveal key mechanisms underlying immune cell-mediated metabolic regulation.
Keywords: Androgens; Estrogens; Intracrine; Lymphocytes; Macrophages; Metabolism.
Copyright © 2018 The Author. Published by Elsevier GmbH.. All rights reserved.
Figures
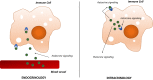
Figure 1
Immune cell intracrinology. In an endocrine model of sex steroid biology, sex steroids are synthesized in classically steroidogenic tissues and disseminated to target cells through the circulation. In contrast, immune cell intracrinology denotes the capacity of immune cells to synthesize, modify, and metabolize sex steroids. Sex steroids signal through nuclear receptors, and a membrane receptor (GPR30) also has been identified for estrogen signaling. Therefore, intracrine function in immune cells can generate sex steroids and their derivatives that mediate intracrine, autocrine, and paracrine effects.
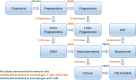
Figure 2
Steroidogenic enzymes involved in the de novo synthesis of sex steroids from cholesterol. Green font indicates that enzyme activity has yet to be demonstrated in immune cells. Orange font indicates that enzyme activity has been demonstrated in macrophages, T lymphocytes, and B lymphocytes. Blue font indicates that enzyme activity has been demonstrated in macrophages and T lymphocytes only.
Figure 3
Schematic of the proposed mechanisms by which sex steroid generation in immune cells could confer metabolic effects. Sex steroids could remain within the cell of origin and act as ligands for cognate nuclear receptors (intracrine signaling). Alternatively, sex steroids could be secreted from the cell and signal through membrane receptors on the same cell (autocrine effects). As sex steroids have demonstrated roles in regulating immune cell differentiation, proliferation, and production of other paracrine mediators, these intracrine and autocrine effects could be key regulators of cellular phenotype and function. Further, secreted sex steroids also could exert signaling effects in neighboring cells (paracrine effects). Estrogens and androgens are important regulators of insulin sensitivity and energy metabolism in key metabolic tissues; thus, tissue resident immune cells could prove an important source of tissue-specific sex steroid production. Adipocytes and other cell types in adipose tissue also express steroidogenic enzymes including aromatase, suggesting that sex steroids could be key signals for cellular crosstalk within metabolic tissues.
Figure 4
Reciprocal regulation of steroidogenic enzymes and other signaling nodes. Cytokines, prostanoids, and growth factors regulate the expression and/or activity of steroidogenic enzymes, and sex steroids, in turn, have been shown to regulate the production of these autocrine and paracrine mediators. Similarly, many nuclear receptors act as both regulators of steroidogenic enzymes and targets of sex steroid signaling. Thus, sex steroid generation may be a central facet of immune cell phenotype and function.
Similar articles
- All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause.
Labrie F. Labrie F. J Steroid Biochem Mol Biol. 2015 Jan;145:133-8. doi: 10.1016/j.jsbmb.2014.06.001. Epub 2014 Jun 9. J Steroid Biochem Mol Biol. 2015. PMID: 24923731 Review. - Intracrine sex steroid synthesis and signaling in human epidermal keratinocytes and dermal fibroblasts.
Pomari E, Dalla Valle L, Pertile P, Colombo L, Thornton MJ. Pomari E, et al. FASEB J. 2015 Feb;29(2):508-24. doi: 10.1096/fj.14-251363. Epub 2014 Nov 12. FASEB J. 2015. PMID: 25392269 - Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery.
Konings G, Brentjens L, Delvoux B, Linnanen T, Cornel K, Koskimies P, Bongers M, Kruitwagen R, Xanthoulea S, Romano A. Konings G, et al. Front Pharmacol. 2018 Sep 19;9:940. doi: 10.3389/fphar.2018.00940. eCollection 2018. Front Pharmacol. 2018. PMID: 30283331 Free PMC article. Review. - Intracrine androgen biosynthesis, metabolism and action revisited.
Schiffer L, Arlt W, Storbeck KH. Schiffer L, et al. Mol Cell Endocrinol. 2018 Apr 15;465:4-26. doi: 10.1016/j.mce.2017.08.016. Epub 2017 Sep 1. Mol Cell Endocrinol. 2018. PMID: 28865807 Free PMC article. Review. - Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology.
Labrie F, Martel C, Bélanger A, Pelletier G. Labrie F, et al. J Steroid Biochem Mol Biol. 2017 Apr;168:9-18. doi: 10.1016/j.jsbmb.2016.12.007. Epub 2017 Jan 30. J Steroid Biochem Mol Biol. 2017. PMID: 28153489 Review.
Cited by
- Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies.
Chakraborty S, Pramanik J, Mahata B. Chakraborty S, et al. Genes Immun. 2021 Jul;22(3):125-140. doi: 10.1038/s41435-021-00139-3. Epub 2021 Jun 14. Genes Immun. 2021. PMID: 34127827 Free PMC article. Review. - Immune and Nervous Systems Interaction in Endocrine Disruptors Toxicity: The Case of Atrazine.
Galbiati V, Buoso E, d'Emmanuele di Villa Bianca R, Paola RD, Morroni F, Nocentini G, Racchi M, Viviani B, Corsini E. Galbiati V, et al. Front Toxicol. 2021 Mar 10;3:649024. doi: 10.3389/ftox.2021.649024. eCollection 2021. Front Toxicol. 2021. PMID: 35295136 Free PMC article. Review. - Beneficial and Deleterious Effects of Female Sex Hormones, Oral Contraceptives, and Phytoestrogens by Immunomodulation on the Liver.
Soria-Jasso LE, Cariño-Cortés R, Muñoz-Pérez VM, Pérez-Hernández E, Pérez-Hernández N, Fernández-Martínez E. Soria-Jasso LE, et al. Int J Mol Sci. 2019 Sep 22;20(19):4694. doi: 10.3390/ijms20194694. Int J Mol Sci. 2019. PMID: 31546715 Free PMC article. Review. - Diet-Induced Obesity Elicits Macrophage Infiltration and Reduction in Spine Density in the Hypothalami of Male but Not Female Mice.
Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, Coss D. Lainez NM, et al. Front Immunol. 2018 Sep 11;9:1992. doi: 10.3389/fimmu.2018.01992. eCollection 2018. Front Immunol. 2018. PMID: 30254630 Free PMC article. - Estrogen, the Peripheral Immune System and Major Depression - A Reproductive Lifespan Perspective.
Engler-Chiurazzi EB, Chastain WH, Citron KK, Lambert LE, Kikkeri DN, Shrestha SS. Engler-Chiurazzi EB, et al. Front Behav Neurosci. 2022 Apr 15;16:850623. doi: 10.3389/fnbeh.2022.850623. eCollection 2022. Front Behav Neurosci. 2022. PMID: 35493954 Free PMC article. Review.
References
- Labrie F. Intracrinology. Molecular and Cellular Endocrinology. 1991;78:C113–C118. - PubMed
- McNamara K.M., Sasano H. The intracrinology of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2015;145:172–178. - PubMed
- Mostaghel E.A., Page S.T., Lin D.W., Fazli L., Coleman I.M., True L.D. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Research. 2007;67:5033–5041. - PubMed
- Cutolo M., Capellino S., Montagna P., Villaggio B., Sulli A., Seriolo B. New roles for estrogens in rheumatoid arthritis. Clinical and Experimental Rheumatology. 2003;21:687–690. - PubMed
- Bélanger C., Luu-The V., Dupont P., Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Hormone and Metabolic Research. 2002;34:737–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous